Qingchang Wenzhong decoction modulates the HIF-1α in CD4+T cells to influence the Warburg effect in mice with ulcerative colitis-associated colorectal cancer
-
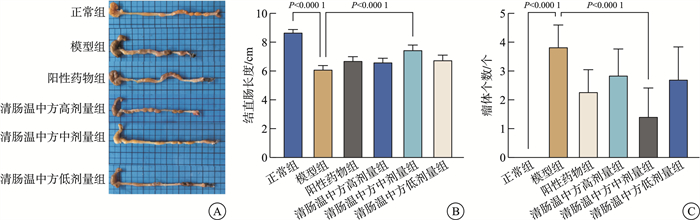
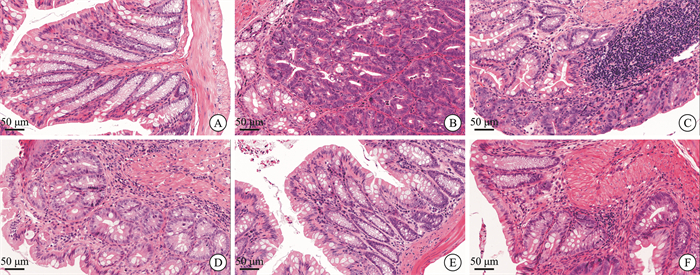
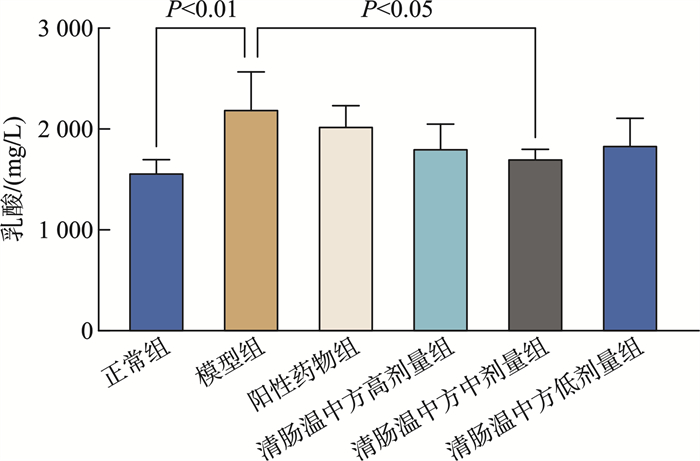
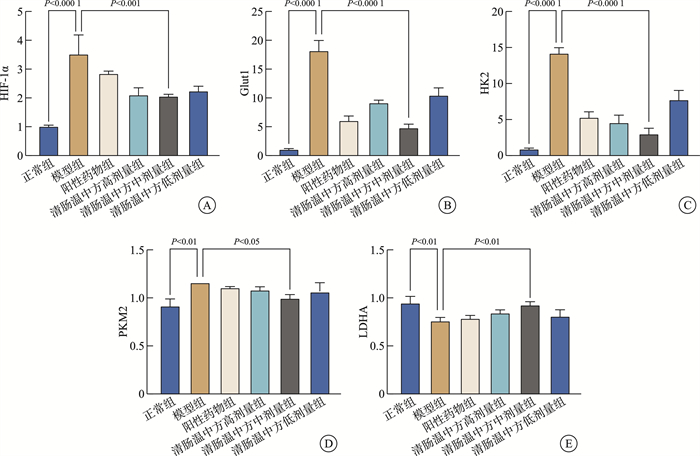
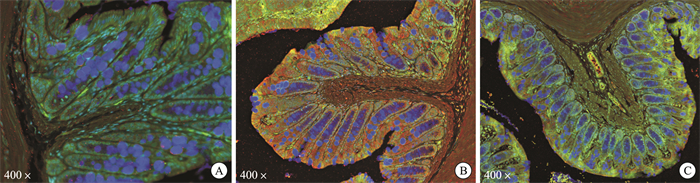
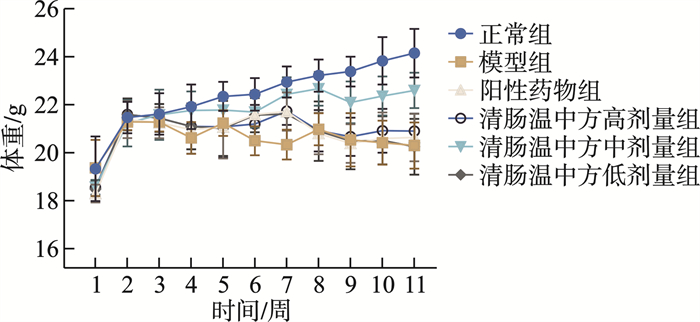
摘要: 目的 观察清肠温中方对溃疡性结肠炎相关性结直肠癌(ulcerative colitis-associated colorectal cancer, UC-CRC)小鼠的整体干预效应, 分析清肠温中方调节CD4+T细胞中HIF-1α对UC-CRC小鼠瓦博格效应的影响。方法 将48只C57BL/6雄性小鼠随机分为正常组、模型组、阳性药物组及清肠温中方高、中、低剂量组, 每组8只。采用诱变剂氧化偶氮甲烷和致炎剂葡聚糖硫酸钠诱导UC-CRC模型。自第2周注射氧化偶氮甲烷开始, 阳性药物组每天予以美沙拉嗪0.04 g/kg剂量灌胃, 清肠温中方高、中、低剂量组每天分别予以18.50、9.25、4.63 g/kg的剂量灌胃, 连续10周。每周测量1次小鼠体重, 检测小鼠血清中乳酸含量、结直肠的长度及瘤体数目; 苏木精-伊红染色法观察小鼠结直肠组织形态学改变; 酶联免疫吸附法检测小鼠结直肠组织中IL-6含量; RT-qPCR法检测小鼠结直肠组织中HIF-lα、Glut1、HK2、PKM2及LDHA的mRNA表达; 免疫荧光双染检测CD4+T细胞中HIF-1α的表达。结果 与正常组比较, 模型组小鼠体重下降, 结直肠长度明显缩短(P < 0.0001), 瘤体数目明显增多(P < 0.0001);血清中乳酸含量显著升高(P < 0.01);黏膜上皮欠完整, 腺体结构紊乱或消失, 隐窝萎缩, 核大而深染, 肿瘤组织形成; 促炎因子IL-6含量明显升高(P < 0.0001);结直肠组织中HIF-1α(P < 0.0001)、Glut1(P < 0.0001)、HK2(P < 0.0001)、PKM2(P < 0.01)及LDHA(P < 0.01)显著升高, CD4+T细胞中HIF-1α明显升高。经清肠温中方干预后小鼠体重下降情况得到改善, 结直肠长度明显延长(P < 0.0001), 瘤体数目显著减少(P < 0.001), 血清中乳酸含量显著下降(P < 0.05), 可见上皮细胞形态较完整, 有效改善了隐窝结构, 减少肿瘤组织的形成; 促炎因子IL-6含量明显下降(P < 0.0001);结直肠组织中HIF-1α(P < 0.001)、G1ut1(P < 0.0001)、HK2(P < 0.0001)、PKM2(P < 0.05)及LDHA(P < 0.01)显著下降, CD4+T细胞中HIF-1α显著降低。结论 清肠温中方一定程度上可以减少UC-CRC模型中瘤体数目, 减轻炎性反应水平, 增加小鼠体重, 其机制可能与HIF-1α调节CD4+T细胞瓦博格效应有关。
-
关键词:
- 清肠温中方 /
- 溃疡性结肠炎相关性结直肠癌 /
- 缺氧诱导因子1α /
- CD4+T细胞 /
- 瓦博格效应
Abstract: Objective To observe the overall intervention effect of Qingchang Wenzhong decoction (QCWZD) on mice of colitis-associated colorectal cancer (UC-CRC) and analyze the impact of QCWZD on the Warburg effect in CD4+T cells via regulating HIF-1α.Methods C57BL/6 male mice were randomly divided into a control group, model group, positive drug group, a high-dose QCWZD group, medium-dose QCWZD group, and low-dose QCWZD group, with 8 mice in each group.The model of UC-CRC was induced by the mutagenic agent azoxymethane (AOM) and the inflammatory agent dextran sulfate sodium (DSS). Since the second week of AOM, the positive drug group has been administered mesalazine at a dose of 0.04 g/kg by gavage daily. In contrast, the high, medi um, and low-dose groups of QCWZD have been administered with doses of 18.50 g/kg, 9.25 g/kg, and 4.63 g/kg by gavage daily, respectively, for a consecutive period of 10 weeks. The body weight of the mice was measured once a week; serum lactic acid content was detected; the length of the colorectal tract and the number of tumors were recorded; histomorphological changes in the colorectal tissue were observed using Hematoxylin and Eosin staining; the levels of interleukin (IL)-6 in the colorectal tissue were measured by enzyme-linked immunosorbent assay(ELISA); the mRNA expression of HIF-lα, Glut1, HK2, PKM2, and LDHA in the colorectal tissue was detected by real-time fluorescence quantitative PCR(RT-qPCR); and the expression of HIF-1α in CD4+T cells was assessed by immunofluorescence double staining.Results Compared with the control group, the model group showed a significant reduction in colorectal length(P < 0.0001) and a marked increase in the number of tumors(P < 0.0001); serum lactic acid content significantly increased(P < 0.01); the mucosal epithelium was incomplete with disordered or absent glandular structures, crypt atrophy, enlarged and hyperchromatic nuclei, and tumor tissue formation; the levels of pro-inflammatory factors IL-6 significantly increased(P < 0.0001); the expression of HIF-1α(P < 0.0001), Glut1(P < 0.0001), HK2(P < 0.0001), PKM2(P < 0.01), and LDHA(P < 0.01) in the colorectal tissue significantly increased, as did HIF-1α in CD4+T cells. After intervention with QCWZD, the colorectal length was significantly extended(P < 0.0001), the number of tumors was significantly reduced(P < 0.001), serum lactic acid content decreased significantly(P < 0.05), and the epithelial cell morphology improved, effectively restoring the crypt structure and reducing tumor formation; the levels of pro-inflammatory factors IL-6 significantly decreased (P < 0.0001); the expression of HIF-1α(P < 0.0001), Glut1(P < 0.0001), HK2(P < 0.0001), PKM2(P < 0.05), and LDHA(P < 0.01) in the colorectal tissue significantly decreased, as did HIF-1α in CD4+ T cells.Conclusion QCWZD can, to some extent, reduce the number of tumors in UC-CRC models, alleviate the level of inflammation, and increase the body weight of mice. The mechanism may be related to the regulation of the Warburg effect in CD4+ T cells by HIF-1α. -

-
表 1 所用引物序列
基因 上游(5'-3') 下游(5'-3') β-Actin CGTTGACATCCGTAAAGACCTC ACAGAGTACTTGCGCTCAGGAG HIF-1α AAGGACAAGTCACCACAGGACA AGGGAGAAAATCAAGTCGTGCT Glut1 TCAACACGGCCTTCACTG CACGATGCTCAGATAGGACATC HK2 TCGCCTGCTTATTCACGGAG CCATCCGGAGTTGACCTCAC PKM2 TGTCTGGAGAAACAGCCAAG CGAATAGCTGCAAGTGGTAGA LDHA CGTCTCCCTGAAGTCTCTTAAC TTCAGCTTGATCACCTCGTAG -
[1] Le berre C, Honap S, Peyrin-biroulet L. Ulcerative colitis[J]. Lancet, 2023, 402(10401): 571-584. doi: 10.1016/S0140-6736(23)00966-2
[2] Shah SC, Itzkowitz SH. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management[J]. Gastroenterology, 2022, 162(3): 715-730. e3. doi: 10.1053/j.gastro.2021.10.035
[3] Wetwittayakhlang P, Golovics PA, Gonczi L, et al. Stable Incidence and Risk Factors of Colorectal Cancer in Ulcerative Colitis: A Population-Based Cohort Between 1977-2020[J]. Clin Gastroenterol Hepatol, 2024, 22(1): 191-193. e3. doi: 10.1016/j.cgh.2023.03.022
[4] Valencia T, Kim JY, Abu-baker S, et al. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis[J]. Cancer Cell, 2014, 26(1): 121-135. doi: 10.1016/j.ccr.2014.05.004
[5] Yin K, Lee J, Liu Z, et al. Mitophagy protein PINK1 suppresses colon tumor growth by metabolic reprogramming via p53 activation and reducing acetyl-CoA production[J]. Cell Death Differ, 2021, 28(8): 2421-2435. doi: 10.1038/s41418-021-00760-9
[6] Warburg O. On the origin of cancer cells[J]. Science, 1956, 123(3191): 309-314. doi: 10.1126/science.123.3191.309
[7] Zhao R, Yang J, Zhai Y, et al. Nucleophosmin 1 promotes mucosal immunity by supporting mitochondrial oxidative phosphorylation and ILC3 activity[J]. Nat Immunol, 2024, 25(9): 1565-1579. doi: 10.1038/s41590-024-01921-x
[8] Reinfeld BI, Madden MZ, Wolf MM, et al. Cell-programmed nutrient partitioning in the tumour microenvironment[J]. Nature, 2021, 593(7858): 282-288. doi: 10.1038/s41586-021-03442-1
[9] Yuan X, Ruan W, Bobrow B, et al. Targeting hypoxia-inducible factors: therapeutic opportunities and challenges[J]. Nat Rev Drug Discov, 2024, 23(3): 175-200. doi: 10.1038/s41573-023-00848-6
[10] Zhang J, Ouyang F, Gao A, et al. ESM1 enhances fatty acid synthesis and vascular mimicry in ovarian cancer by utilizing the PKM2-dependent warburg effect within the hypoxic tumor microenvironment[J]. Mol Cancer, 2024, 23(1): 94. doi: 10.1186/s12943-024-02009-8
[11] Lin Y, Xue K, Li Q, et al. Cyclin-Dependent Kinase 7 Promotes Th17/Th1 Cell Differentiation in Psoriasis by Modulating Glycolytic Metabolism[J]. J Invest Dermatol, 2021, 141(11): 2656-2667. e11. doi: 10.1016/j.jid.2021.04.018
[12] Sun Z, Li J, Wang W, et al. Qingchang Wenzhong Decoction Accelerates Intestinal Mucosal Healing Through Modulation of Dysregulated Gut Microbiome, Intestinal Barrier and Immune Responses in Mice[J]. Front Pharmacol, 2021, 12: 738152. doi: 10.3389/fphar.2021.738152
[13] 孙中美, 陈晓伟, 胡立明, 等. 清肠温中方对缓解期溃疡性结肠炎的远期作用研究[J]. 中国中西医结合消化杂志, 2021, 29(9): 619-623. https://zxyxh.whuhzzs.com/article/doi/10.3969/j.issn.1671-038X.2021.09.05
[14] Cheng Y, Li J, Zhang X, et al. Protective Effect of Qingchang Wenzhong Decoction on Colitis and Colitis-Related Carcinogenesis by Regulating Inflammation and Intestinal Fibrosis[J]. J Inflamm Res, 2023, 16: 1479-1495. doi: 10.2147/JIR.S402395
[15] 王木源, 李军祥, 卢心毓, 等. 清肠温中方对溃疡性结肠炎小鼠组织驻留记忆CD4+T细胞的调控作用[J]. 中国中西医结合消化杂志, 2023, 31(8): 583-588. https://zxyxh.whuhzzs.com/article/doi/10.3969/j.issn.1671-038X.2023.08.02
[16] 王志斌, 陈晨, 郭一, 等. 清肠温中方治疗轻中度溃疡性结肠炎的临床研究[J]. 中国中西医结合杂志, 2018, 38(1): 15-19.
[17] Tanaka T, Kohno H, Suzuki R, et al. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate[J]. Cancer Sci, 2003, 94(11): 965-973. doi: 10.1111/j.1349-7006.2003.tb01386.x
[18] Fang JS, Gillies RD, Gatenby RA. Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression[J]. Semin Cancer Biol, 2008, 18(5): 330-337. doi: 10.1016/j.semcancer.2008.03.011
[19] Chapman NM, Boothby MR, Chi H. Metabolic coordination of T cell quiescence and activation[J]. Nat Rev Immunol, 2020, 20(1): 55-70. doi: 10.1038/s41577-019-0203-y
[20] Mcnamee EN, Korns JD, Homann D, et al. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function[J]. Immunol Res, 2013, 55(1-3): 58-70. doi: 10.1007/s12026-012-8349-8
[21] Dang EV, Barbi J, Yang HY, et al. Control of T(H)17/T(reg)balance by hypoxia-inducible factor 1[J]. Cell, 2011, 146(5): 772-784. doi: 10.1016/j.cell.2011.07.033
[22] Shi LZ, Wang R, Huang G, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells[J]. J Exp Med, 2011, 208(7): 1367-1376. doi: 10.1084/jem.20110278
[23] Wang Y, Li M, Zha A. mTOR promotes an inflammatory response through the HIF1 signaling pathway in ulcerative colitis[J]. Int Immunopharmacol, 2024, 134: 112217. doi: 10.1016/j.intimp.2024.112217
-

计量
- 文章访问数: 148
- 施引文献: 0



 下载:
下载: