The effect of Qingchang Wenzhong Decoction on the AhR/IL-22 signaling pathway in mice with ulcerative colitis
-
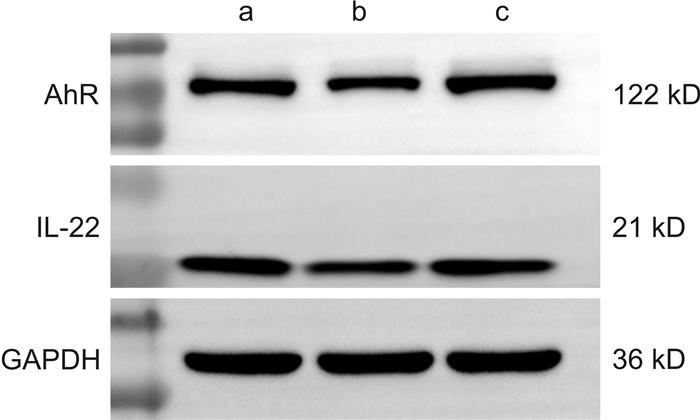
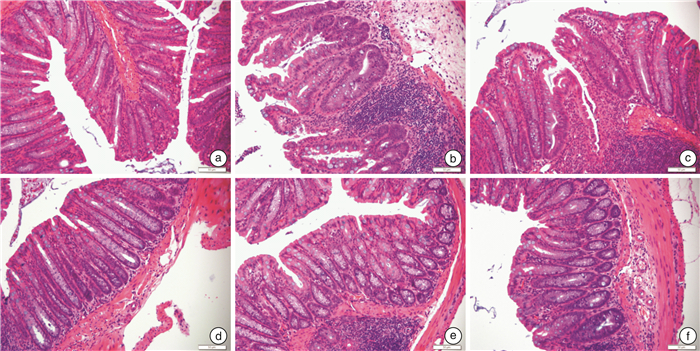
摘要: 目的 研究清肠温中方对葡聚糖硫酸钠(DSS)诱导的溃疡性结肠炎(UC)小鼠AhR/IL-22信号通路的影响。方法 将28只SPF级健康雌性C57BL/6小鼠随机分为空白组、模型组、清肠温中方低剂量组、清肠温中方中剂量组、清肠温中方高剂量组、美沙拉秦组。空白组小鼠全程自由进食饮水,模型组和清肠温中方低、中、高各剂量组及美沙拉秦组均采用自由饮用2.5%(w/v)DSS溶液7 d复制UC模型,第8天开始模型组给予去离子水灌胃,清肠温中方各剂量组给予相应浓度的中药干预1周,美沙拉秦组给予美沙拉秦水溶液干预1周。期间每日观察小鼠一般情况,测量体重、检测便潜血、记录粪便性状,计算疾病活动指数(DAI)。干预结束后留取结肠组织,Western Blot检测AhR、IL-22蛋白水平,RT-qPCR测定AhR、IL-22mRNA的相对表达量。结果 与空白组比较,UC小鼠呈现明显的肠道炎症,表现出不同程度的便血、腹泻、体重下降等,清肠温中方中剂量组和美沙拉秦组小鼠体重较模型组明显升高(P<0.05);清肠温中方中剂量组小鼠较模型组DAI下降明显(P<0.05)。清肠温中方组AhR、IL-22蛋白表达量较模型组升高明显(P<0.05);清肠温中方组AhR、IL-22mRNA表达量较模型组显著升高(P<0.01)。结论 清肠温中方可能通过激活UC小鼠中AhR/IL-22信号通路,达到治疗UC的目的。
-
关键词:
- 溃疡性结肠炎 /
- 清肠温中方 /
- AhR/IL-22信号通路 /
- 肠黏膜修复
Abstract: Objective To study the effect of Qingchang Wenzhong Decoction(QCWZD) on dextran sodium sulfate(DSS)-induced AhR/IL-22 signaling pathway in mice with ulcerative colitis(UC).Methods Twenty-eight healthy female C57BL/6 mice were randomly divided into control group, DSS group, QCWZD low-dose group, QCWZD medium-dose group, QCWZD high-dose group, Mesalazine group. Mice in control group were fed and watered freely throughout the whole process. DSS group, doses group of QCWZD and Mesalazine group were used to replicate the UC model by freely drinking 2.5 %(w/v) DSS solution for 7 days. On day 8, the model group was given deionized water for gavage, and each dose of the QCWZD was given the corresponding concentration of Chinese medicine for 1 week in DSS group, and the Mesalazine solution for 1 week in the Mesalazine group. During this period, the mice were observed daily for general condition, weight measurement, detection of fecal occult blood, recording of fecal properties, and calculation of disease activity index(DAI). At the end of the intervention, colonic tissues were collected and the levels of AhR and IL-22 protein were measured, and the levels of AhR/IL-22mRNA were determined.Results Compared with control group, UC mice showed obvious intestinal inflammation, exhibiting different degrees of blood in stool, diarrhea and weight loss, etc. The body weight of mice in QCWZD medium-dose group and Mesalazine group was significantly higher than that in DSS group(P<0.05); the DAI of mice in the medium-dose group of QCWZD decreased significantly compared with that in DSS group(P<0.05). The expressions of AhR and IL-22 protein in QCWZD group were significantly higher than those in DSS group(P<0.05); the expressions of AhR/IL-22mRNA in QCWZD group were significantly higher than those in DSS group(P<0.01).Conclusion QCWZD maybe used to treat UC by activating the AhR/IL-22 signaling pathway in UC mice. -

-
表 1 DAI的评分标准[13]
评分项目 0 1 2 3 4 体重下降指数 无下降 >1%~5% >5%~10% >10%~15% >15% 粪便性状 正常 / 松散 / 稀便 粪便潜血 无 / 阳性 / 肉眼血便 表 2 本实验所用引物
基因 上游(5’-3’) 下游(5’-3’) GAPDH CTTCCAGCCTTCCTTCCTTGG AATGCCTGGGTACATGGTGG AhR GAGCACAAATCAGAGACTGG TGGAGGAAGCATAGAAGACC IL-22 TCCGAGGAGTCAGTGCTAAA AGAACGTCTTCCAGGGTGAA 表 3 清肠温中方对各组小鼠体重的影响
%,X±S 组别 例数 体重变化 空白组 5 106.527±1.539 模型组 5 98.215±2.9591) 清肠温中方低剂量组 4 100.599±1.598 清肠温中方中剂量组 5 103.066±2.5952) 清肠温中方高剂量组 4 101.874±2.208 美沙拉秦组 5 103.139±2.3132) 与空白组比较,1)P<0.01;与模型组比较,2)P<0.05。 表 4 清肠温中方对各组小鼠DAI评分的影响
分,X±S 组别 例数 DAI评分 空白组 5 0.000±0.000 模型组 5 0.800±0.6911) 清肠温中方低剂量组 4 0.333±0.385 清肠温中方中剂量组 5 0.000±0.0002) 清肠温中方高剂量组 4 0.167±0.333 美沙拉秦组 5 0.133±0.298 与空白组比较,1)P<0.05;与模型组比较,2)P<0.05。 表 5 清肠温中方对小鼠结肠组织AhR、IL-22蛋白表达的影响
X±S 组别 例数 AhR IL-22 空白组 5 0.737±0.031 0.713±0.050 模型组 5 0.453±0.0671) 0.433±0.1012) 清肠温中方组 5 0.737±0.0403) 0.683±0.1104) 与空白组比较,1)P<0.01,2)P<0.05;与模型组比较,3)P<0.01,4)P<0.05。 表 6 清肠温中方对小鼠结肠组织AhR、IL-22 mRNA的影响
X±S 组别 例数 AhR mRNA IL-22 mRNA 空白组 5 1.006±0.106 1.026±0.251 模型组 5 1.375±0.076 1.000±0.106 清肠温中方组 5 2.348±0.3691) 1.552±0.1741) 与模型组比较,1)P<0.01。 -
[1] 李军祥, 陈誩. 溃疡性结肠炎中西医结合诊疗共识意见(2017年)[J]. 中国中西医结合消化杂志, 2018, 26(2): 105-111, 120. https://zxyxh.whuhzzs.com/article/doi/10.3969/j.issn.1671-038X.2018.02.01
[2] Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders[J]. J Crohns Colitis, 2017, 11(6): 649-670. doi: 10.1093/ecco-jcc/jjx008
[3] Raine T, Bonovas S, Burisch J, et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment[J]. J Crohns Colitis, 2022, 16(1): 2-17. doi: 10.1093/ecco-jcc/jjab178
[4] Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults[J]. Gut, 2019, 68(Suppl 3): s1-s106. doi: 10.1136/gutjnl-2019-318484
[5] Hazel K, O'Connor A. Emerging treatments for inflammatory bowel disease[J]. Ther Adv Chronic Dis, 2020, 11: 2040622319899297.
[6] Renga G, Nunzi E, Pariano M, et al. Optimizing therapeutic outcomes of immune checkpoint blockade by a microbial tryptophan metabolite[J]. J Immunother Cancer, 2022, 10(3): e003725. doi: 10.1136/jitc-2021-003725
[7] Li YY, Wang XJ, Su YL, et al. Baicalein ameliorates ulcerative colitis by improving intestinal epithelial barrier via AhR/IL-22 pathway in ILC3s[J]. Acta Pharmacol Sin, 2022, 43(6): 1495-1507. doi: 10.1038/s41401-021-00781-7
[8] Yan S, Wang P, Wei H, et al. Treatment of ulcerative colitis with Wu-Mei-Wan by inhibiting intestinal inflammatory response and repairing damaged intestinal mucosa[J]. Phytomedicine, 2022, 105: 154362. doi: 10.1016/j.phymed.2022.154362
[9] 毛堂友, 程佳伟, 魏仕兵, 等. 清肠温中方治疗溃疡性结肠炎84例[J]. 环球中医药, 2016, 9(4): 479-481. doi: 10.3969/j.issn.1674-1749.2016.04.029
[10] 王志斌, 陈晨, 郭一, 等. 清肠温中方治疗轻中度溃疡性结肠炎的临床研究[J]. 中国中西医结合杂志, 2018, 38(1): 15-19. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZXJ201801004.htm
[11] Sun Z, Li J, Wang W, et al. Qingchang Wenzhong Decoction Accelerates Intestinal Mucosal Healing Through Modulation of Dysregulated Gut Microbiome, Intestinal Barrier and Immune Responses in Mice[J]. Front Pharmacol, 2021, 12: 738152. doi: 10.3389/fphar.2021.738152
[12] 王木源, 李军祥, 卢心毓, 等. 清肠温中方对溃疡性结肠炎小鼠组织驻留记忆CD4+T细胞的调控作用[J]. 中国中西医结合消化杂志, 2023, 31(8): 583-588. https://zxyxh.whuhzzs.com/article/doi/10.3969/j.issn.1671-038X.2023.08.02
[13] Sun Z, Li J, Dai Y, et al. Indigo Naturalis Alleviates Dextran Sulfate Sodium-Induced Colitis in Rats via Altering Gut Microbiota[J]. Front Microbiol, 2020, 11: 731. doi: 10.3389/fmicb.2020.00731
[14] Hou Q, Ye L, Liu H, et al. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22[J]. Cell Death Differ, 2018, 25(9): 1657-1670. doi: 10.1038/s41418-018-0070-2
[15] Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology[J]. Pharmacol Rev, 2015, 67(2): 259-279. doi: 10.1124/pr.114.009001
[16] Meynier M, Baudu E, Rolhion N, et al. AhR/IL-22 pathway as new target for the treatment of post-infectious irritable bowel syndrome symptoms[J]. Gut Microbes, 2022, 14(1): 2022997. doi: 10.1080/19490976.2021.2022997
[17] Li J, Doty A, Glover SC. Aryl hydrocarbon receptor signaling involves in the human intestinal ILC3/ILC1 conversion in the inflamed terminal ileum of Crohn's disease patients[J]. Inflamm Cell Signal, 2016, 3(3): e1404.
[18] Geng S, Cheng S, Li Y, et al. Faecal Microbiota Transplantation Reduces Susceptibility to Epithelial Injury and Modulates Tryptophan Metabolism of the Microbial Community in a Piglet Model[J]. J Crohns Colitis, 2018, 12(11): 1359-1374.
[19] Lindemans CA, Calafiore M, Mertelsmann AM, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration[J]. Nature, 2015, 528(7583): 560-564. doi: 10.1038/nature16460
[20] Monteleone I, Rizzo A, Sarra M, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract[J]. Gastroenterology. 2011, 141(1): 237-248, 248. e1. doi: 10.1053/j.gastro.2011.04.007
[21] de Araújo EF, Loures FV, Preite NW, et al. AhR Ligands Modulate the Differentiation of Innate Lymphoid Cells and T Helper Cell Subsets That Control the Severity of a Pulmonary Fungal Infection[J]. Front Immunol, 2021, 12: 630938. doi: 10.3389/fimmu.2021.630938
-




 下载:
下载: