Research advances on anti-ulcerative colitis effect of natural compounds through pyroptosis
-
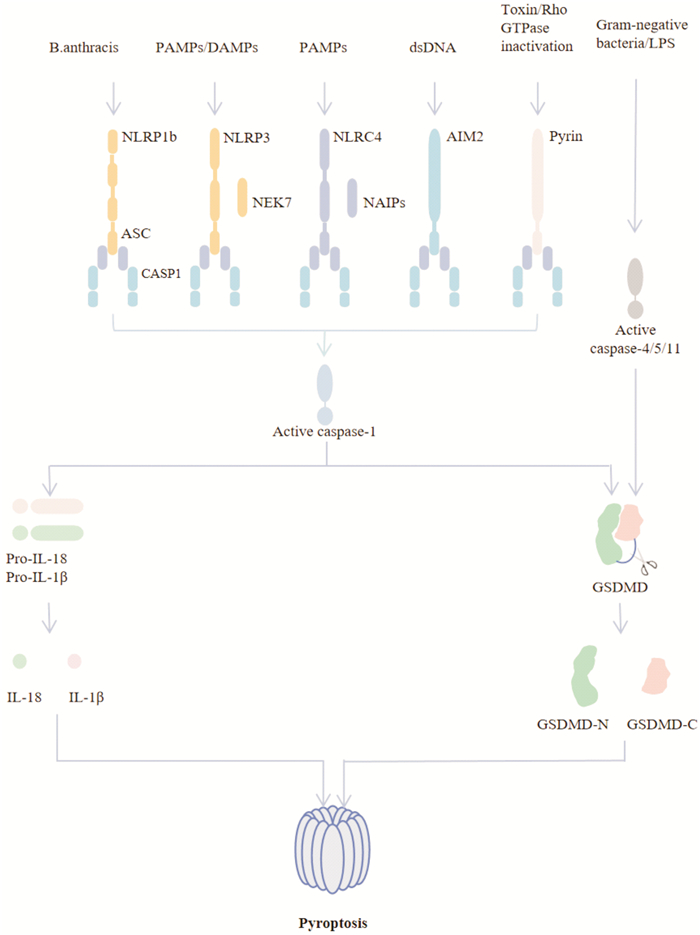
摘要: 细胞焦亡是近年来发现的一种有别于细胞凋亡、铁死亡和自噬的新型程序性细胞死亡方式。细胞焦亡依赖于半胱氨酸天冬氨酸蛋白水解酶,通过激活炎症小体切割GSDM关键蛋白,诱导促炎细胞因子IL-1β和IL-18成熟并释放到细胞外环境中,产生级联炎症反应。溃疡性结肠炎是一种慢性非特异性炎症性疾病,具有反复发作、缠绵难愈等特点。大量研究表明,细胞焦亡与溃疡性结肠炎的发生、发展关系密切。研究细胞焦亡在溃疡性结肠炎筛选标志物、诊断疾病、发掘治疗新靶点等方面至关重要。基于此,本文总结了细胞焦亡发生、发展的作用机制,归纳了天然化合物通过介导细胞焦亡通路抗溃疡性结肠炎的作用,以期为今后探索细胞焦亡的机制和天然化合物治疗溃疡性结肠炎提供新思路。Abstract: Pyroptosis is newly discovered type of programmed cell death that is different from apoptosis, ferroptosis, and autophagy. Pyroptosis is mediated by caspase and commonly induced by the gasdermin(GSDM) family and is accompanied by the release of inflammatory cytokines such as IL-1β and IL-18. Ulcerative colitis is a chronic, non-specific inflammatory disease characterized by recurrent attacks and persistent healing. Numerous studies have shown that pyroptosis is closely related to the occurrence and development of ulcerative colitis. Studying pyroptosis is essential to ulcerative colitisin screening markers, diagnosing diseases, and discovering new therapeutic targets. Based on this, this article summarizes the mechanism of the occurrence and development of pyroptosis and generalizes the role of natural compounds in anti-ulcerative colitis by mediating the pyroptosis pathway, in order to provide new ideas for exploring the mechanism of pyroptosis and natural compounds in the treatment of ulcerative colitis in the future.
-
Key words:
- pyroptosis /
- ulcerative colitis /
- natural compounds /
- research advances
-

-
表 1 天然化合物通过细胞焦亡途径抗UC机制总结
类别 名称 来源 调节焦亡机制 参考文献 生物碱类 EVO 吴茱萸 抑制NLRP3、ASC和caspase-1表达,降低结肠组织中IL-1β分泌 [32] 青藤碱 青藤根 抑制NOD-、LRR-和NLRP3炎症小体的激活 [33] PAL 紫苏、黄连和延胡索等 抑制NLRP3炎症小体,通过Nrf2途径减轻氧化应激和炎症反应 [37-38] OPAL 黄藤素氧化代谢产物 激活Nrf2通路,显著抑制NLRP3炎症小体的激活 [39] 木脂素类 五味子甲素 五味子 降低IL-6、IL-1β、TNF-α和IFN-γ的水平,降低NF-κB和NOS的表达 [41] 五味子乙素 五味子 激活依赖AMPK/NRF2信号转导的ROS诱导的线粒体损伤;抑制NLRP3炎症小体激活介导的IL-1β水平,抑制细胞焦亡 [42] 黄酮类 山姜素 姜科植物 下调TLR4、NF-κB和NLRP3炎症小体 [43] 忍冬苷 金银花 抑制NLRP3炎症小体的激活 [44] 木犀草素 金银花、紫苏等木犀草科植物 阻断IL-17A信号而有效地抑制NLRP3的表达 [45] 汉黄芩苷 黄芩 抑制txnip依赖的NF-κB通路及NLRP3炎症小体的形成和激活 [46] 刺芒柄花素 黄芪等刺芒柄花类 降低NLRP3通路蛋白(NLRP3、ASC、IL-1β)水平 [47] 萜类 MunronoidⅠ 地黄连 促进K48连接的泛素化和NLRP3降解,抑制NLRP3炎症小体的焦亡 [49] 雷公藤多苷 雷公藤 抑制NOxs活性和ROS的产生及NLRP3炎症小体、ASC和caspase-1的激活 [50-51] 人参皂苷Rg1 人参 通过上调NLRP12的表达,抑制IL-1β和TNF-α的释放 [52] 人参皂苷Rk3 人参 降低TNF-α、IL-1β、IL-6、NLRP3、ASC及caspase-1的表达,阻断NLRP3炎症小体通路 [53] 青蒿烯 青蒿素衍生物 抑制mtROS产生,阻止NLRP3激活,抑制IL-1β的产生;阻断NLRC4和AIM2炎症小体介导的IL-1β分泌和IL-6的产生 [54] SM934 新型水溶性青蒿素类似物 通过NLRP3/NF-κB/MAPK信号轴抑制上皮细胞焦亡 [55] 多酚类 连翘提取物 连翘 通过Nrf-NLRP3通路介导细胞焦亡 [56] APE 苹果 抑制NLRP3、ASC、caspase-1、GSDM D、GSDM D-N和IL-1β的表达,降低IL-1β、IL-18表达 [57] WEL 墨旱莲等蟛蜞菊 抑制NLRP3的激活和caspase-1磷酸化,减少IL-1β的释放 [58] 姜黄素 姜黄 抑制NLRP3炎症小体激活和IL-1β产生 [59] BUR 姜黄素衍生物 促进NLRP3炎症小体失活,增强抗炎反应 [60] 酚酸类 SA 蔬果、谷物和油料作物以及葡萄酒和醋等 降低TNF-α、IL-1β、IL-6、IL-8、IL-17α和干扰素-γ的表达,抑制NLRP3激活 [61] 中药提取物 茜草乙醇提取物 茜草 抑制NLRP3的形成,抑制IL-6/JAK2/STAT3的激活 [62] 槐耳水提取物 槐耳 抑制NLRP3激活诱导的IL-1β分泌和caspase-1裂解;通过自噬溶酶体途径促进NLRP3降解,降低NLRP3蛋白的表达 [63] 其他 海南萝芙木的果胶多糖提取物 海南萝芙木 调节NF-κB通路和IL-17,抑制caspase-1和IL-1β的表达 [64] 烟曲霉素C 烟曲霉 抑制TNF-α、IL-1β和IL-17A和mRNA表达;抑制NLRP3激活,降低caspase-1表达 [65] 嗜黏蛋白阿克曼菌 益生菌 上调NLRP3、caspase-1和IL-1β的表达 [66] GPA肽 鱼皮明胶水解物 增强AMPK磷酸化,抑制ROS的产生,促进NLRP3蛋白降解,抑制NLRP3激活 [69] 产乳酸益生菌酿酒酵母 产乳酸益生菌酿酒酵母 抑制NLRP3及caspase-1通路的激活 [70] -
[1] Broz P, Pelegrin P, Shao F. The gasdermins, a protein family executing cell death and inflammation[J]. Nat Rev Immunol, 2020, 20(3): 143-157. doi: 10.1038/s41577-019-0228-2
[2] Sarrio D, Martinez-Val J, Molina-Crespo A, et al. The multifaceted roles of gasdermins in cancer biology and oncologic therapies[J]. Biochim Biophys Acta Rev Cancer, 2021, 1876(2): 188635. doi: 10.1016/j.bbcan.2021.188635
[3] Ruan JW, Wang SJ, Wang JB. Mechanism and regulation of pyroptosis-mediated in cancer cell death[J]. Chem Biol Interact, 2020, 323: 109052. doi: 10.1016/j.cbi.2020.109052
[4] Zhou CB, Fang JY. The role of pyroptosis in gastrointestinal cancer and immune responses to intestinal microbial infection[J]. Biochim Biophys Acta BBA Rev Cancer, 2019, 1872(1): 1-10. doi: 10.1016/j.bbcan.2019.05.001
[5] Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages[J]. Nature, 1992, 358(6382): 167-169. doi: 10.1038/358167a0
[6] Monack DM, Raupach B, Hromockyj AE, et al. Salmonella typhimurium invasion induces apoptosis in infected macrophages[J]. Proc Natl Acad Sci USA, 1996, 93(18): 9833-9838. doi: 10.1073/pnas.93.18.9833
[7] Hilbi H, Moss JE, Hersh D, et al. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB[J]. J Biol Chem, 1998, 273(49): 32895-32900. doi: 10.1074/jbc.273.49.32895
[8] Cookson BT, Brennan MA. Pro-inflammatory programmed cell death[J]. Trends Microbiol, 2001, 9(3): 113-114.
[9] Burdette BE, Esparza AN, Zhu H, et al. Gasdermin D in pyroptosis[J]. Acta Pharm Sin B, 2021, 11(9): 2768-2782. doi: 10.1016/j.apsb.2021.02.006
[10] Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages[J]. Cell Microbiol, 2006, 8(11): 1812-1825. doi: 10.1111/j.1462-5822.2006.00751.x
[11] Wu CQ, Lu W, Zhang Y, et al. Inflammasome activation triggers blood clotting and host death through pyroptosis[J]. Immunity, 2019, 50(6): 1401-1411.e4. doi: 10.1016/j.immuni.2019.04.003
[12] Shi JJ, Zhao Y, Wang YP, et al. Inflammatory caspases are innate immune receptors for intracellular LPS[J]. Nature, 2014, 514(7521): 187-192. doi: 10.1038/nature13683
[13] 刘吉祥, 王一冲, 孔佑甲, 等. 基于NLRP3炎症小体探讨中医药防治结直肠癌的相关进展[J]. 中国中西医结合消化杂志, 2022, 30(10): 747-752. https://zxyxh.whuhzzs.com/article/doi/10.3969/j.issn.1671-038X.2022.10.13
[14] 张海明, 梁凤霞, 杨胜兰, 等. 基于NLRP3炎症小体探讨中医药防治溃疡性结肠炎的机制[J]. 中国中西医结合消化杂志, 2021, 29(7): 524-528. doi: 10.3969/j.issn.1671-038X.2021.07.17 https://zxyxh.whuhzzs.com/article/doi/10.3969/j.issn.1671-038X.2021.07.17
[15] Ross C, Chan AH, von Pein J, et al. Dimerization and auto-processing induce caspase-11 protease activation within the non-canonical inflammasome[J]. Life Sci Alliance, 2018, 1(6): e201800237. doi: 10.26508/lsa.201800237
[16] Winkler S, Rosen-Wolff A. Caspase-1: an integral regulator of innate immunity[J]. Semin Immunopathol, 2015, 37(4): 419-427. doi: 10.1007/s00281-015-0494-4
[17] Shi JJ, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death[J]. Nature, 2015, 526(7575): 660-665. doi: 10.1038/nature15514
[18] Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling[J]. Nature, 2015, 526(7575): 666-671. doi: 10.1038/nature15541
[19] Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling[J]. Nat Rev Immunol, 2016, 16(7): 407-420. doi: 10.1038/nri.2016.58
[20] Ruhl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+)efflux[J]. Eur J Immunol, 2015, 45(10): 2927-2936. doi: 10.1002/eji.201545772
[21] 吴开春, 阴玥, 万健, 等. 溃疡性结肠炎认识新视角[J]. 中国中西医结合消化杂志, 2023, 31(3): 163-167, 172. doi: 10.3969/j.issn.1671-038X.2023.03.02 https://zxyxh.whuhzzs.com/article/doi/10.3969/j.issn.1671-038X.2023.03.02
[22] Short SP, Pilat JM, Barrett CW, et al. Colonic epithelial-derived selenoprotein P is the source for antioxidant-mediated protection in colitis-associated cancer[J]. Gastroenterology, 2021, 160(5): 1694-1708.e3. doi: 10.1053/j.gastro.2020.12.059
[23] Ma YZ, Jiang JX, Gao Y, et al. Research progress of the relationship between pyroptosis and disease[J]. Am J Transl Res, 2018, 10(7): 2213-2219.
[24] Zhang JJ, Wirtz S. Does pyroptosis play a role in inflammasome-related disorders?[J]. Int J Mol Sci, 2022, 23(18): 10453. doi: 10.3390/ijms231810453
[25] Ning YM, Lin K, Fang J, et al. Pyroptosis-related signature predicts the progression of ulcerative colitis and colitis-associated colorectal cancer as well as the anti-TNF therapeutic response[J]. J Immunol Res, 2023, 2023: 7040113.
[26] Kantono M, Guo BC. Inflammasomes and cancer: the dynamic role of the inflammasome in tumor development[J]. Front Immunol, 2017, 8: 1132. doi: 10.3389/fimmu.2017.01132
[27] Liu X, Zhang ZB, Ruan JB, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores[J]. Nature, 2016, 535(7610): 153-158. doi: 10.1038/nature18629
[28] Zaki MH, Lamkanfi M, Kanneganti TD. The Nlrp3 inflammasome: contributions to intestinal homeostasis[J]. Trends Immunol, 2011, 32(4): 171-179. doi: 10.1016/j.it.2011.02.002
[29] Chen GY, Nunez G. Inflammasomes in intestinal inflammation and cancer[J]. Gastroenterology, 2011, 141(6): 1986-1999. doi: 10.1053/j.gastro.2011.10.002
[30] Zanoni I, Tan YH, di Gioia M, et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells[J]. Science, 2016, 352(6290): 1232-1236. doi: 10.1126/science.aaf3036
[31] Nowarski R, Jackson R, Gagliani N, et al. Epithelial IL-18 equilibrium controls barrier function in colitis[J]. Cell, 2015, 163(6): 1444-1456. doi: 10.1016/j.cell.2015.10.072
[32] Shen P, Zhang ZC, Zhu KP, et al. Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-κB and NLRP3 inflammasome[J]. Biomedecine Pharmacother, 2019, 110: 786-795. doi: 10.1016/j.biopha.2018.12.033
[33] Zhou Y, Chen S, Gu WX, et al. Sinomenine hydrochloride ameliorates dextran sulfate sodium-induced colitis in mice by modulating the gut microbiota composition whilst suppressing the activation of the NLRP3 inflammasome[J]. Exp Ther Med, 2021, 22(5): 1287. doi: 10.3892/etm.2021.10722
[34] Kumar P, Srivastava V, Chaturvedi R, et al. Elicitor enhanced production of protoberberine alkaloids from in vitro cell suspension cultures of Tinospora cordifolia(Willd.)Miers ex Hook. F, Thoms[J]. Plant Cell Tiss Organ Cult, 2017, 130(2): 417-426. doi: 10.1007/s11240-017-1237-0
[35] Zhang Q, Chen C, Wang FQ, et al. Simultaneous screening and analysis of antiplatelet aggregation active alkaloids from rhizoma corydalis[J]. Pharm Biol, 2016, 54(12): 3113-3120. doi: 10.1080/13880209.2016.1211714
[36] Sun ML, Xu LJ, Peng YL, et al. Multiscale analysis of the contents of palmatine in the Nature populations of Phellodendron amurense in Northeast China[J]. J For Res, 2016, 27(2): 265-272. doi: 10.1007/s11676-015-0200-3
[37] Mai CT, Wu MM, Wang CL, et al. Palmatine attenuated dextran sulfate sodium(DSS)-induced colitis via promoting mitophagy-mediated NLRP3 inflammasome inactivation[J]. Mol Immunol, 2019, 105: 76-85. doi: 10.1016/j.molimm.2018.10.015
[38] Zhang XJ, Yuan ZW, Qu C, et al. Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota[J]. Pharmacol Res, 2018, 137: 34-46. doi: 10.1016/j.phrs.2018.09.010
[39] Cheng JJ, Ma XD, Zhang HT, et al. 8-Oxypalmatine, a novel oxidative metabolite of palmatine, exhibits superior anti-colitis effect via regulating Nrf2 and NLRP3 inflammasome[J]. Biomedecine Pharmacother, 2022, 153: 113335. doi: 10.1016/j.biopha.2022.113335
[40] 邢楠楠, 屈怀东, 任伟超, 等. 五味子主要化学成分及现代药理作用研究进展[J]. 中国实验方剂学杂志, 2021, 27(15): 210-218. doi: 10.13422/j.cnki.syfjx.20211407
[41] Zhang WF, Yang Y, Su X, et al. Deoxyschizandrin suppresses dss-induced ulcerative colitis in mice[J]. Saudi J Gastroenterol, 2016, 22(6): 448-455. doi: 10.4103/1319-3767.195552
[42] Zhang WW, Wang WS, Shen CZ, et al. Network pharmacology for systematic understanding of Schisandrin B reduces the epithelial cells injury of colitis through regulating pyroptosis by AMPK/Nrf2/NLRP3 inflammasome[J]. Aging(Albany NY), 2021, 13(19): 23193-23209.
[43] He XX, Wei ZK, Wang JJ, et al. Alpinetin attenuates inflammatory responses by suppressing TLR4 and NLRP3 signaling pathways in DSS-induced acute colitis[J]. Sci Rep, 2016, 6: 28370. doi: 10.1038/srep28370
[44] Lv Q, Xing Y, Liu J, et al. Lonicerin targets EZH2 to alleviate ulcerative colitis by autophagy-mediated NLRP3 inflammasome inactivation[J]. Acta Pharm Sin B, 2021, 11(9): 2880-2899. doi: 10.1016/j.apsb.2021.03.011
[45] Lin TJ, Yin SY, Hsiao PW, et al. Transcriptomic analysis reveals a controlling mechanism for NLRP3 and IL-17A in dextran sulfate sodium(DSS)-induced colitis[J]. Sci Rep, 2018, 8(1): 14927. doi: 10.1038/s41598-018-33204-5
[46] Liu Q, Zuo R, Wang K, et al. Oroxindin inhibits macrophage NLRP3 inflammasome activation in DSS-induced ulcerative colitis in mice via suppressing TXNIP-dependent NF-κB pathway[J]. Acta Pharmacol Sin, 2020, 41(6): 771-781. doi: 10.1038/s41401-019-0335-4
[47] Wu DC, Wu KY, Zhu QT, et al. Formononetin administration ameliorates dextran sulfate sodium-induced acute colitis by inhibiting NLRP3 inflammasome signaling pathway[J]. Mediators Inflamm, 2018, 2018: 3048532.
[48] Ge YH, Zhang JX, Mu SZ, et al. Munronoids A-J, ten new limonoids from Munronia unifoliolata Oliv[J]. Tetrahedron, 2012, 68(2): 566-572. doi: 10.1016/j.tet.2011.11.003
[49] Ma XY, Di QQ, Li XL, et al. Munronoid I ameliorates DSS-induced mouse colitis by inhibiting NLRP3 inflammasome activation and pyroptosis Via modulation of NLRP3[J]. Front Immunol, 2022, 13: 853194. doi: 10.3389/fimmu.2022.853194
[50] 钦丹萍, 孙佩娜, 周毅骏, 等. 雷公藤多苷对溃疡性结肠炎大鼠模型炎症及TLR4/MyD88信号通路的作用[J]. 中华医学杂志, 2016, 96(18): 1444-1449. doi: 10.3760/cma.j.issn.0376-2491.2016.18.012
[51] Ma FX, Ke YF, Zhong JH, et al. Effect of Tripterygium wilfordii polycoride on the NOXs-ROS-NLRP3 inflammasome signaling pathway in mice with ulcerative colitis[J]. Evid Based Complement Alternat Med, 2019, 2019: 9306283.
[52] Zhu G, Wang HN, Wang TC, et al. Ginsenoside Rg1 attenuates the inflammatory response in DSS-induced mice colitis[J]. Int Immunopharmacol, 2017, 50: 1-5. doi: 10.1016/j.intimp.2017.06.002
[53] Tian M, Ma P, Zhang Y, et al. Ginsenoside Rk3 alleviated DSS-induced ulcerative colitis by protecting colon barrier and inhibiting NLRP3 inflammasome pathway[J]. Int Immunopharmacol, 2020, 85: 106645. doi: 10.1016/j.intimp.2020.106645
[54] Hua L, Liang SL, Zhou YH, et al. Artemisinin-derived artemisitene blocks ROS-mediated NLRP3 inflammasome and alleviates ulcerative colitis[J]. Int Immunopharmacol, 2022, 113(Pt B): 109431.
[55] Shao MJ, Yan YX, Zhu FH, et al. Artemisinin analog SM934 alleviates epithelial barrier dysfunction via inhibiting apoptosis and caspase-1-mediated pyroptosis in experimental colitis[J]. Front Pharmacol, 2022, 13: 849014. doi: 10.3389/fphar.2022.849014
[56] Chao LM, Lin J, Zhou J, et al. Polyphenol rich Forsythia suspensa extract alleviates DSS-induced ulcerative colitis in mice through the Nrf2-NLRP3 pathway[J]. Antioxidants(Basel), 2022, 11(3): 475.
[57] Liu F, Wang XJ, Cui Y, et al. Apple polyphenols extract(APE)alleviated dextran sulfate sodium induced acute ulcerative colitis and accompanying neuroinflammation via inhibition of apoptosis and pyroptosis[J]. Foods, 2021, 10(11): 2711. doi: 10.3390/foods10112711
[58] Wei WC, Ding ML, Zhou K, et al. Protective effects of wedelolactone on dextran sodium sulfate induced murine colitis partly through inhibiting the NLRP3 inflammasome activation via AMPK signaling[J]. Biomedecine Pharmacother, 2017, 94: 27-36. doi: 10.1016/j.biopha.2017.06.071
[59] Gong ZZ, Zhao SN, Zhou JF, et al. Curcumin alleviates DSS-induced colitis via inhibiting NLRP3 inflammsome activation and IL-1β production[J]. Mol Immunol, 2018, 104: 11-19. doi: 10.1016/j.molimm.2018.09.004
[60] Zhang JF, Li QM, Zhang X, et al. Bisdemethoxycurcumin alleviates dextran sodium sulfate-induced colitis via inhibiting NLRP3 inflammasome activation and modulating the gut microbiota in mice[J]. Antioxidants(Basel), 2022, 11(10): 1994.
[61] Qian B, Wang CQ, Zeng Z, et al. Ameliorative effect of sinapic acid on dextran sodium sulfate-(DSS-)induced ulcerative colitis in Kunming(KM)mice[J]. Oxid Med Cell Longev, 2020, 2020: 8393504.
[62] Qin W, Luo H, Yang L, et al. Rubia cordifolia L. ameliorates DSS-induced ulcerative colitis in mice through dual inhibition of NLRP3 inflammasome and IL-6/JAK2/STAT3 pathways[J]. Heliyon, 2022, 8(8): e10314. doi: 10.1016/j.heliyon.2022.e10314
[63] Wang LJ, Yu ZX, Wei C, et al. Huaier aqueous extract protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NLRP3 inflammasome activation[J]. Oncotarget, 2017, 8(20): 32937-32945. doi: 10.18632/oncotarget.16513
[64] Cui LJ, Yuan W, Chen FY, et al. Pectic polysaccharides ameliorate the pathology of ulcerative colitis in mice by reducing pyroptosis[J]. Ann Transl Med, 2022, 10(6): 347. doi: 10.21037/atm-22-877
[65] Guo WJ, Hu SS, Elgehama A, et al. Fumigaclavine C ameliorates dextran sulfate sodium-induced murine experimental colitis via NLRP3 inflammasome inhibition[J]. J Pharmacol Sci, 2015, 129(2): 101-106. doi: 10.1016/j.jphs.2015.05.003
[66] Qu SW, Fan LN, Qi YD, et al. Akkermansia muciniphila alleviates dextran sulfate sodium(DSS)-induced acute colitis by NLRP3 activation[J]. Microbiol Spectr, 2021, 9(2): e0073021. doi: 10.1128/Spectrum.00730-21
[67] Zheng LF, Wei HK, Yu HC, et al. Fish skin gelatin hydrolysate production by ginger powder induces glutathione synthesis to prevent hydrogen peroxide induced intestinal oxidative stress via the Pept1-p62-Nrf2 cascade[J]. J Agric Food Chem, 2018, 66(44): 11601-11611. doi: 10.1021/acs.jafc.8b02840
[68] Zheng L, Yu H, Wei H, et al. Antioxidative peptides of hydrolysate prepared from fish skin gelatin using ginger protease activate antioxidant response element-mediated gene transcription in IPEC-J2 cells[J]. J Funct Foods, 2018, 51: 104-112. doi: 10.1016/j.jff.2018.08.033
[69] Deng Z, Ni JJ, Wu XY, et al. GPA peptide inhibits NLRP3 inflammasome activation to ameliorate colitis through AMPK pathway[J]. Aging(Albany NY), 2020, 12(18): 18522-18544.
[70] Sun SY, Xu XX, Liang L, et al. Lactic acid-producing probiotic Saccharomyces cerevisiae attenuates ulcerative colitis via suppressing macrophage pyroptosis and modulating gut microbiota[J]. Front Immunol, 2021, 12: 777665. doi: 10.3389/fimmu.2021.777665
-




 下载:
下载: