Effects of somatostatin combined with rhubarb on intestinal mucosal barrier and microbial diversity in patients with acute pancreatitis
-
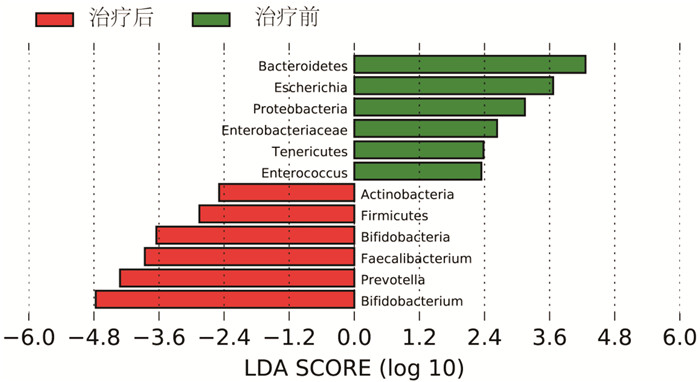
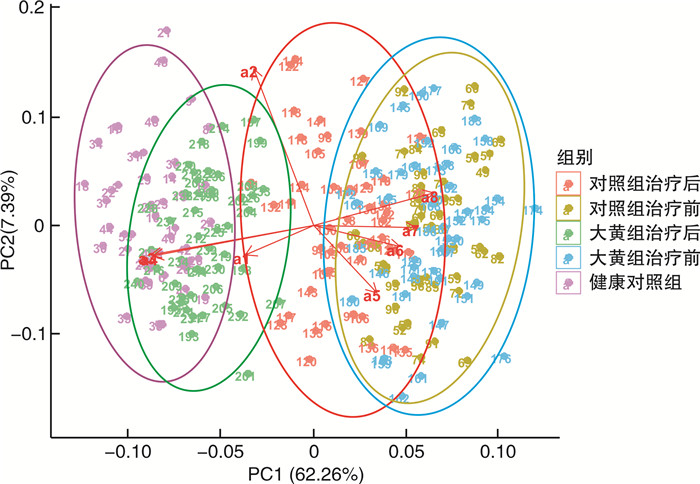
摘要: 目的 探讨生长抑素联合大黄对急性胰腺炎患者的疗效及对肠道黏膜屏障和菌群多样性的影响。方法 选择2021年1月—2022年6月期间就诊的96例急性胰腺炎患者为研究对象,按照随机数字表法将所有患者分为大黄组(n=48)和对照组(n=48)。将同期就诊的健康体检人群作为健康对照组(n=48)。所有患者均在基础治疗的基础上予以生长抑素静脉推注,大黄组在此基础上加用生大黄饮剂。比较两组患者的疗效,同时于入院时及治疗后7 d时收集大黄组、对照组及健康对照组大便标本,对肠道菌群情况进行检测,分析治疗前后两组患者的大便菌群变化情况以及与健康对照组之间的关系。同时对治疗前后两组患者的肠道黏膜屏障功能进行评估并分析之间的差异。结果 治疗后1、3及7 d时,大黄组患者的急性生理与慢性健康Ⅱ(acute physiology and chronic health evaluation Ⅱ,APACHEⅡ)评分、淀粉酶、白细胞及C反应蛋白均低于对照组(P < 0.05)。大黄组患者的腹痛、腹胀消失时间及住院天数均少于对照组,转ICU及手术占比均低于对照组(P < 0.05)。治疗后7 d时,大黄组患者的二胺氧化酶、D-乳酸及细菌内毒素均低于对照组(P < 0.05);Chao1指数、ACE指数及Shannon指数均高于对照组,而Simpson指数低于对照组(P < 0.05)。两组患者干预前的菌群特征差异无统计学意义,干预后两组菌群均发生显著改变,且大黄组患者的菌群向健康对照组靠近的趋势较对照组更加明显。大黄组患者治疗前肠道优势菌群为拟杆菌门、埃希氏杆菌属、变形菌门、肠杆菌科、厚壁菌门及肠球菌,而治疗后7 d优势菌群转化为放线菌门、厚壁菌门、双歧杆菌、栖粪杆菌属、普氏菌及双歧杆菌属(LDA>2log10)。结论 大黄联合生长抑素能够通过改善肠道微生态环境,从而促进急性胰腺炎患者的病情更快缓解。Abstract: Objective To investigate the effects of somatostatin combined with rhubarb on intestinal mucosal barrier and microbial diversity in patients with acute pancreatitis.Methods From January 2021 to June 2022, 96 patients with acute pancreatitis who visited our hospital were selected as research objects, all patients were divided into rhubarb group(n=48) and control group(n=48) according to the random number table method. In addition, the healthy people who visited the physical examination center of our hospital during the same period were selected as the healthy control group(n=48). All patients were given somatostatin intravenous injection on the basis of basic treatment according to the guidelines, and rhubarb group was given raw rhubarb drink on the basis of this. The efficacy of the two groups was compared. At the same time, stool samples of the rhubarb group, control group and healthy control group were collected at admission and seven days after treatment to detect the intestinal flora, and the changes of stool flora of the two groups of patients before and after treatment and the relationship between the two groups and the healthy control group were analyzed. At the same time, intestinal mucosal barrier function was evaluated and the difference between the two groups before and after treatment was analyzed.Results The acute physiology and chronic health evaluation Ⅱ(APACHEⅡ) score, amylase, leukocyte and C-reactive protein in rhubarb group were lower than those in the control group at one, three, and seven days after treatment(P < 0.05). The disappearance time of abdominal pain and abdominal distension and hospitalization days in rhubarb group were lower than those in the control group, and the proportion of ICU transfer and operation were lower than those in the control group(P < 0.05). The DOx, D-L and bacterial endotoxin in rhubarb group were lower than those in the control group at seven days after treatment(P < 0.05). On the day seven after treatment, Chao1 index, ACE index and Shannon index in rhubarb group were higher than those in the control group, while Simpson index was lower than those in control group(P < 0.05). There was no significant difference in the microflora characteristics between the two groups before intervention, but the microflora of the two groups changed significantly, and the trend of the microflora of the rhubarb group was more obvious than that of the control group after intervention. Before treatment, the dominant intestinal bacteria in the rhubarb group were Bacteroidetes, Escherichia, Proteobacteria, Enterobacteriaceae, Tenericutes, Enterococcus. After seven days of treatment, the dominant bacteria were transformed into Actnobacteria, Firmicutes, Bifidobacteria, Faecalibacterium, Prevotella, Bifidobacterium(LDA>2log10).Conclusion Rhubarb combined with somatostatin can improve the intestinal microecological environment and promote the faster remission of AP patients.
-
Key words:
- acute pancreatitis /
- somatostatin /
- rhubarb /
- intestinal flora /
- intestinal mucosal barrier
-

-
表 1 两组AP患者的临床资料比较
X±S,例(%) 临床资料 大黄组(n=48) 对照组(n=48) t/χ2 P 年龄/岁 47.23±6.78 46.55±7.04 0.482 0.631 性别 0.711 0.399 男 32(66.67) 28(58.33) 女 16(33.33) 20(41.67) BMI 24.78±2.04 24.33±2.77 0.906 0.367 发病时间/h 12.43±3.04 13.11±4.23 0.904 0.368 吸烟史 24(50.00) 27(56.25) 0.367 0.539 饮酒史 15(31.25) 13(27.08) 0.202 0.653 高血压 15(31.25) 13(27.08) 0.301 0.584 糖尿病 9(18.75) 7(14.58) 0.394 0.661 使用抗生素 32(66.67) 34(70.83) 0.884 0.385 镇痛药物 0.071 0.789 氯诺昔康 37(77.08) 33(68.75) 盐酸哌滴定 9(18.75) 8(16.67) 病情 0.211 0.646 轻症 34(70.83) 36(75.00) 重症 14(29.17) 12(25.00) 病因 0.479 0.923 胆源性 31(64.58) 30(62.50) 高甘油三酯 2(4.17) 1(2.08) 酒精性 9(18.75) 10(20.83) 其他 6(12.50) 7(14.58) 表 2 两组AP患者各时间点APACHE Ⅱ评分、淀粉酶等指标的比较
X±S 组别 例数 时间 APACHE Ⅱ评分/分 淀粉酶/(U/L) 白细胞/(×109/L) C反应蛋白/(mg/L) 大黄组 48 基线 11.04±3.13 873.24±104.27 17.52±4.21 93.43±14.59 治疗后1 d 8.12±2.73 744.33±121.47 15.04±3.08 70.53±10.24 治疗后3 d 6.24±1.03 432.13±79.83 11.09±1.97 35.19±8.04 治疗后7 d 3.75±0.94 147.24±52.13 7.34±2.19 16.23±4.32 对照组 48 基线 11.78±3.42 862.49±123.47 17.84±4.09 94.22±15.37 治疗后1 d 9.92±2.61 803.27±102.49 16.52±3.04 76.37±11.76 治疗后3 d 7.85±1.73 499.54±81.55 13.43±2.87 39.87±7.11 治疗后7 d 4.83±0.47 179.54±62.13 9.01±1.88 19.52±4.94 组间比较 F/P 9.516/ < 0.001 12.839/ < 0.001 6.523/ < 0.001 17.433/ < 0.001 时间比较 F/P 172.481/ < 0.001 1 207.429/ < 0.001 219.955/ < 0.001 1 157.953/ < 0.001 交互比较 F/P 6.973/ < 0.001 17.522/ < 0.001 9.347/ < 0.001 24.563/ < 0.001 表 3 两组AP患者腹痛、腹胀消失时间等指标的比较
X±S,例(%) 组别 例数 腹痛消失时间/d 腹胀消失时间/d 住院天数/d 转ICU 手术 大黄组 48 4.14±0.97 7.23±1.22 14.17±4.21 2(4.17) 1(2.08) 对照组 48 5.03±1.02 8.49±1.47 16.99±4.52 8(16.67) 6(12.50) t/χ2 4.381 4.569 3.162 4.019 3.947 P < 0.001 < 0.001 0.002 0.045 0.049 表 4 两组AP患者治疗前及治疗后7 d时肠道屏障功能比较
X±S 组别 例数 时间 DOx/(IU/L) D-L/(μg/L) 细菌内毒素/(pg/mL) 大黄组 48 治疗前 18.24±4.13 11.18±2.21 53.47±7.98 治疗后7 d 6.78±1.74 4.21±1.77 13.47±2.98 对照组 48 治疗前 17.83±4.02 11.04±2.83 52.09±9.12 治疗后7 d 8.33±1.55 5.99±2.37 16.47±3.22 组间比较 F/P 11.592/ < 0.001 7.984/ < 0.001 14.593/ < 0.001 时间比较 F/P 74.592/ < 0.001 69.437/ < 0.001 104.599/ < 0.001 交互比较 F/P 8.247/ < 0.001 4.987/ < 0.001 11.951/ < 0.001 表 5 两组AP患者治疗前及治疗后7 d时粪便微生物群α多样性的比较
组别 例数 时间 菌群丰度 菌群多样性 Chao1指数 ACE指数 Shannon指数 Simpson指数 大黄组 48 治疗前 319.49±98.53 309.57±92.49 3.14±0.69 0.12±0.04 治疗后7 d 237.49±73.52 214.59±63.77 1.73±0.39 0.22±0.05 对照组 48 治疗前 324.59±103.49 317.43±95.47 3.11±0.78 0.11±0.03 治疗后7 d 192.53±63.59 180.33±52.49 1.55±0.27 0.25±0.06 组间比较 F/P 5.743/ < 0.001 4.375/0.007 5.032/ < 0.001 3.784/0.013 时间比较 F/P 39.433/ < 0.001 38.574/ < 0.001 79.842/ < 0.001 84.327/ < 0.001 交互比较 F/P 3.524/0.037 3.251/0.045 4.634/0.003 4.022/0.012 -
[1] 朱国玲, 陈朔华, 樊学东, 等. 基线BMI水平对急性胰腺炎发病风险影响的前瞻性队列研究[J]. 中华流行病学杂志, 2021, 42(12): 2131-2137. doi: 10.3760/cma.j.cn112338-20201027-01286
[2] Boxhoorn L, Voermans RP, Bouwense SA, et al. Acute pancreatitis[J]. Lancet, 2020, 396(10252): 726-734. doi: 10.1016/S0140-6736(20)31310-6
[3] Li XY, He C, Zhu Y, et al. Role of gut microbiota on intestinal barrier function in acute pancreatitis[J]. World J Gastroenterol, 2020, 26(18): 2187-2193. doi: 10.3748/wjg.v26.i18.2187
[4] Wang ZJ, Li F, Liu J, et al. Intestinal microbiota-an unmissable bridge to severe acute pancreatitis-associated acute lung injury[J]. Front Immunol, 2022, 13: 913178. doi: 10.3389/fimmu.2022.913178
[5] Zhu XD, Duan FX, Zhang Y, et al. Acadesine alleviates acute pancreatitis-related lung injury by mediating the barrier protective function of pulmonary microvascular endothelial cells[J]. Int Immunopharmacol, 2022, 111: 109165. doi: 10.1016/j.intimp.2022.109165
[6] Zhao HB, Jia L, Yan QQ, et al. Effect of Clostridium butyricum and butyrate on intestinal barrier functions: study of a rat model of severe acute pancreatitis with intra-abdominal hypertension[J]. Front Physiol, 2020, 11: 561061. doi: 10.3389/fphys.2020.561061
[7] 满意, 常加伟, 汤亲青. 重症急性胰腺炎不同病程的细菌移位及肠道菌群组成结构的变化研究[J]. 中国急救医学, 2019, 39(1): 71-76.
[8] 中华医学会外科学分会胰腺外科学组. 中国急性胰腺炎诊治指南(2021)[J]. 中华外科杂志, 2021, 59(7): 578-587. https://www.cnki.com.cn/Article/CJFDTOTAL-SYNK201307012.htm
[9] 中华医学会, 中华医学会杂志社, 中华医学会消化病学分会, 等. 急性胰腺炎基层诊疗指南(实践版·2019)[J]. 中华全科医师杂志, 2019, 18(9): 954-959.
[10] 宋轶, 刘杰, 路晓光, 等. 大黄附子汤改善重症急性胰腺炎大鼠肠道动力障碍的机制研究[J]. 中华急诊医学杂志, 2021, 30(8): 954-959.
[11] Gao ZM, Sui JD, Fan R, et al. Emodin protects against acute pancreatitis-associated lung injury by inhibiting NLPR3 inflammasome activation via Nrf2/HO-1 signaling[J]. Drug Des Devel Ther, 2020, 14: 1971-1982. doi: 10.2147/DDDT.S247103
[12] 韦艳萍, 黄勤英, 廖帅, 等. 重症急性胰腺炎继发感染危险因素及外周血炎症指标预测价值[J]. 中华医院感染学杂志, 2021, 31(15): 2332-2336. https://www.cnki.com.cn/Article/CJFDTOTAL-ZHYY202115019.htm
[13] Yu SS, Wu D, Jin K, et al. Low serum ionized calcium, elevated high-sensitivity C-reactive protein, neutrophil-lymphocyte ratio, and body mass index(BMI)are risk factors for severe acute pancreatitis in patients with hypertriglyceridemia pancreatitis[J]. Med Sci Monit, 2019, 25: 6097-6103.
[14] Cai YL, Wang SQ, Zhong HJ, et al. The effect of anemia on the severity and prognosis of patients with acute pancreatitis: a single-center retrospective study[J]. Medicine, 2022, 101(52): e32501.
[15] Zhang QK, Hu FL, Guo FY, et al. Emodin attenuates adenosine triphosphate-induced pancreatic ductal cell injury in vitro via the inhibition of the P2X7/NLRP3 signaling pathway[J]. Oncol Rep, 2019, 42(4): 1589-1597.
[16] Tong HF, Huang Z, Chen H, et al. Emodin reverses gemcitabine resistance of pancreatic cancer cell lines through inhibition of IKKβ/NF-κB signaling pathway[J]. Onco Targets Ther, 2020, 13: 9839-9848.
[17] Carver W, Fix E, Fix C, et al. Effects of emodin, a plant-derived anthraquinone, on TGF-β1-induced cardiac fibroblast activation and function[J]. J Cell Physiol, 2021, 236(11): 7440-7449.
[18] Yin J, Zhao XS, Chen XJ, et al. Emodin suppresses hepatocellular carcinoma growth by regulating macrophage polarization via microRNA-26a/transforming growth factor beta 1/protein kinase B[J]. Bioengineered, 2022, 13(4): 9548-9563.
[19] Xian MH, Cai JL, Zheng KN, et al. Aloe-emodin prevents nerve injury and neuroinflammation caused by ischemic stroke via the PI3K/AKT/mTOR and NF-κB pathway[J]. Food Funct, 2021, 12(17): 8056-8067.
[20] Mandal B, Mondal HK, Das S. In situ eactivity of electrochemically generated semiquinone on Emodin and its Cu/Mn omplexes with pyrimidine based nucleic acid bases and calf thymus DNA: insight into free radical induced cytotoxicity of anthracyclines[J]. Biochem Biophys Res Commun, 2019, 515(3): 505-509.
[21] Li QQ, Gao J, Pang XH, et al. Molecular mechanisms of action of emodin: As an anti-cardiovascular disease drug[J]. Front Pharmacol, 2020, 11: 559607.
[22] Guo RM, Li YJ, Han M, et al. Emodin attenuates acute lung injury in Cecal-ligation and puncture rats[J]. Int Immunopharmacol, 2020, 85: 106626.
[23] Ren CX, Wang YJ, Lin XF, et al. A combination of formic acid and monolaurin attenuates enterotoxigenicEscherichia coliinduced intestinal inflammation in piglets by inhibiting the NF-κB/MAPK pathways with modulation of gut microbiota[J]. J Agric Food Chem, 2020, 68(14): 4155-4165.
[24] Kong LM, Deng J, Zhou X, et al. Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury[J]. Cell Death Dis, 2021, 12(10): 928.
[25] Layunta E, Latorre E, Grasa L, et al. Intestinal serotonergic system is modulated by Toll-like receptor 9[J]. J Physiol Biochem, 2022, 78(3): 689-701.
[26] Cohen-Kedar S, Keizer D, Schwartz S, et al. Commensal fungi and their cell-wall β-glucans direct differential responses in human intestinal epithelial cells[J]. Eur J Immunol, 2021, 51(4): 864-878.
[27] Wang WB, Li JT, Hui Y, et al. Combination of pseudoephedrine and emodin ameliorates LPS-induced acute lung injury by regulating macrophage M1/M2 polarization through the VIP/cAMP/PKA pathway[J]. Chin Med, 2022, 17(1): 19.
[28] 郭静, 尚海, 马丽炎, 等. 芦荟大黄素衍生物AE-YJ通过PI3K-Akt/NF-κB和MAPK/NF-κB途径抑制LPS诱导RAW264.7细胞炎症介质的释放[J]. 中国药理学通报, 2021, 37(12): 1700-1708.
[29] van den Berg FF, van Dalen D, Hyoju SK, et al. Western-type diet influences mortality from necrotising pancreatitis and demonstrates a central role for butyrate[J]. Gut, 2021, 70(5): 915-927.
-




 下载:
下载: