Effect of modified Banxia Xiexin Decoction on gastrointestinal motility, brain-gut peptide and neurohormone secretion factor of patients with functional dyspepsia
-
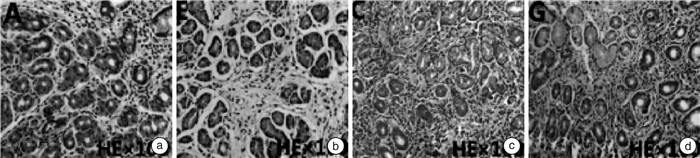
摘要: 目的 探讨半夏泻心汤加味方对功能性消化不良患者胃肠动力及脑肠肽、神经激素分泌因子的影响。方法 前瞻性纳入2019年12月—2021年12月收治的126例功能性消化不良患者为研究对象,以抽签法分为研究组和对照组,各63例。对照组给予多潘立酮片治疗,研究组在对照组基础上加用半夏泻心汤加味方治疗,均持续治疗4周。比较两组治疗后的总有效率、消化不良症状指数(symptom index of dyspepsia,SID)、尼平消化不良指数(nepean dyspepsia index,NDI),治疗前后的病理变化情况、餐后胃电图参数、胃肠动力指标及脑肠肽水平差异,并通过RT-PCR法对胃黏膜组织中胃促生长素、胆囊收缩素mRNA的表达进行观察,统计两组患者的不良反应发生情况。结果 研究组的治疗总有效率为96.83%,高于对照组的82.54%,差异有统计学意义(P < 0.05)。两组患者治疗前后胃镜下未发现明显异常改变。苏木精-伊红染色结果显示,两组患者治疗前局部胃黏膜可见水肿、充血及炎性改变,治疗后,两组患者的胃组织形态恢复正常,未发生肠上皮化生、出血及增生等改变。研究组治疗后SID评分、NDI评分改善程度较对照组更佳,差异有统计学意义(P < 0.05)。治疗后,两组患者的餐后胃电参数均改善,且研究组餐后1导联和2导联频率、振幅、餐后/餐前功率及RA均明显优于对照组(P < 0.05)。治疗后,两组患者的血清胃肠激素、胃动素水平均明显升高(P < 0.05),且研究组的胃肠激素、胃动素水平显著高于对照组(P < 0.05)。治疗后,两组患者的血清神经肽Y、神经降压素水平均显著降低(P < 0.05),且研究组更低(P < 0.05)。治疗后,两组患者的胃黏膜组织中胆囊收缩素mRNA表达水平均显著降低,胃促生长素mRNA水平升高,研究组较对照组改善情况更优,差异有统计学意义(P < 0.05)。两组患者在治疗期间均未出现明显的不良反应。结论 半夏泻心汤加味方可有效改善功能性消化不良患者的临床症状和胃肠动力,调节脑肠肽、神经激素分泌因子水平,其作用机制可能与调节胃促生长素、胆囊收缩素的表达相关。Abstract: Objective To explore the effects of modified Banxia Xiexin Decoction on gastrointestinal motility, brain-gut peptides and neurohormone secretion factors in patients with functional dyspepsia.Methods One hundred and twenty-six patients with functional dyspepsia admitted from December 2019 to December 2021 were divided into a study group and a control group by balloting, 63 cases in each one. The patients in the control group were treated with domperidone tablets, while those in the research group were additionally treated with modified Banxia Xiexin Decoction on the basis of the control group. All the treatment lasted for four weeks. The total effective rate, symptom index of dyspepsia (SID), nepean dyspepsia index(NDI), pathological changes before and after treatment, postprandial electrogastrogram parameters, gastrointestinal motility indicators and brain-gut peptide levels between the two groups were compared. The mRNA expressions of ghrelin andcholecystokinin(CCK) in gastric mucosa were observed by RT-PCR, and the adverse reactions between the two groups were counted.Results The effective rate was 96.83% in the study group and 82.54% in the control group(P < 0.05). No obvious abnormal changes were found under gastroscopy before and after treatment. HE staining results showed that edema, congestion and inflammatory changes were observed in the local gastric mucosa of the two groups before treatment. After treatment, the gastric morphology of the two groups of patients returned to normal, without intestinal metaplasia, bleeding and hyperplasia. The improvement of SID score and NDI score in the study group was better than that in the control group after treatment(P < 0.05). After treatment, the mean of postprandial gastric electrical parameters in both groups were improved, and the postprandial one lead and two lead frequency, amplitude, postprandial/preprandial power and RA in the study group were significantly better than those in the control group(P < 0.05). After treatment, serum gastrointestinal hormone and motilin in two groups were significantly increased(P < 0.05), and the levels of gastrointestinal hormone and motilin in the research group were significantly higher than those in the control group(P < 0.05). After treatment, serum neu-ropeptide Y and neurotensin levels were significantly decreased in two groups(P < 0.05), and lower in the study group(P < 0.05). After treatment, CCK mRNA expression level in gastric mucosa group was significantly decreased and ghrelin mRNA level was increased in both groups, and the improvement in the study group was better than that in the control group(P < 0.05). There were no obvious adverse reactions during treatment in both groupsConclusion Banxia Xiexin Decoction can effectively improve the clinical symptoms and gastrointestinal motility of patients with functional dyspepsia, and regulate the levels of brain-gut peptide and neurohormone secretion factors, whose mechanism of action may be related to the expression of ghrelin and CCK.
-

-
表 1 两组患者临床资料比较例,X±S
组别 例数 性别 年龄/岁 病程/月 BMI 男 女 对照组 63 33 30 27.53±3.24 23.84±3.12 23.04±3.25 研究组 63 36 27 28.41±5.78 22.97±3.36 22.26±3.31 χ2/t 0.288 1.054 1.506 1.335 P 0.591 0.294 0.135 0.184 表 2 两组患者的临床疗效比较
例(%) 组别 例数 痊愈 显效 有效 无效 总有效 对照组 63 21(33.33) 18(28.57) 13(20.63) 11(17.46) 52(82.54) 研究组 63 35(55.56) 16(25.40) 10(15.87) 2(3.17) 61(96.83) χ2 6.948 P 0.008 表 3 两组患者治疗前后SID评分和NDI评分比较
分,X±S 组别 例数 SID t P NDI t P 治疗前 治疗后 治疗前 治疗后 对照组 63 21.15±1.53 18.43±2.08 9.283 < 0.001 87.83±5.61 64.23±4.22 26.864 < 0.001 研究组 63 20.87±1.62 13.91±3.58 14.059 < 0.001 88.12±5.43 58.03±5.18 31.825 < 0.001 t 0.997 8.665 0.295 7.365 P 0.321 < 0.001 0.769 < 0.001 表 4 两组患者餐后1导联胃电图参数比较X±S
组别 例数 频率/cpm 振幅/μV 餐后/餐前功率 RA/(μV·s) 对照组 63 3.02±0.15 293.08±5.76 1.42±0.36 62.83±6.62 研究组 63 3.14±0.18 222.64±15.49 1.55±0.32 60.54±6.07 t 4.065 33.831 2.142 2.024 P < 0.001 < 0.001 0.034 0.045 表 5 两组患者餐后2导联胃电图参数比较
X±S 组别 例数 频率/cpm 振幅/μV 餐后/餐前功率 RA/(μV·s) 对照组 63 3.13±0.14 321.94±4.91 1.48±0.27 63.21±9.23 研究组 63 3.28±0.06 318.56±8.62 1.65±0.33 60.64±7.38 t 7.817 2.704 3.165 36.490 P < 0.001 0.008 0.002 < 0.001 表 6 两组患者治疗前后胃肠动力指标水平比较
ng/L,X±S 组别 例数 GAS t P MTL t P 治疗前 治疗后 治疗前 治疗后 对照组 63 31.85±8.04 40.88±6.75 6.827 < 0.001 289.83±43.36 331.76±34.25 6.023 < 0.001 研究组 63 32.01±7.93 46.41±8.15 10.051 < 0.001 290.51±34.65 348.31±11.58 12.558 < 0.001 t 0.590 4.148 0.097 3.633 P 0.556 < 0.001 0.923 < 0.001 表 7 两组患者治疗前后脑肠肽水平比较
pg/mL,X±S 组别 例数 NPY t P NT t P 治疗前 治疗后 治疗前 治疗后 对照组 63 91.81±12.73 76.51±8.82 7.841 < 0.001 114.78±10.93 84.01±11.53 15.373 < 0.001 研究组 63 92.27±12.64 70.04±11.61 10.480 < 0.001 115.12±11.05 69.22±8.67 25.939 < 0.001 t 0.204 3.552 0.174 8.138 P 0.839 0.001 0.862 0.001 表 8 两组患者的胃黏膜组织CCK、ghrelin mRNA表达水平比较
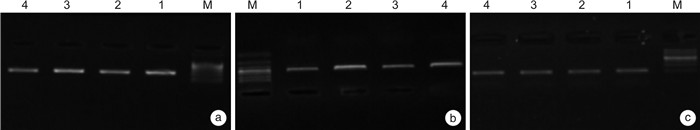
X±S 组别 例数 CCK mRNA t P ghrelin mRNA t P 治疗前 治疗后 治疗前 治疗后 对照组 63 0.82±0.05 0.71±0.01 17.123 < 0.001 0.49±0.02 0.72±0.01 81.642 < 0.001 研究组 63 0.83±0.01 0.63±0.02 70.993 < 0.001 0.51±0.08 0.83±0.02 113.589 < 0.001 t 1.557 28.397 1.926 39.046 P 0.122 < 0.001 0.057 < 0.001 -
[1] Ford AC, Mahadeva S, Carbone MF, et al. Functional dyspepsia[J]. Lancet, 2020, 396(10263): 1689-1702. doi: 10.1016/S0140-6736(20)30469-4
[2] Wauters L, Talley NJ, Walker MM, et al. Novel concepts in the pathophysiology and treatment of functional dyspepsia[J]. Gut, 2020, 69(3): 591-600. doi: 10.1136/gutjnl-2019-318536
[3] Madisch A, Andresen V, Enck P, et al. The diagnosis and treatment of functional dyspepsia[J]. Dtsch Arztebl Int, 2018, 115(13): 222-232.
[4] Kim BJ, Kuo B. Gastroparesis and functional dyspepsia: a blurring distinction of pathophysiology and treatment[J]. J Neurogastroenterol Motil, 2019, 25(1): 27-35. doi: 10.5056/jnm18162
[5] 《中成药治疗优势病种临床应用指南》标准化项目组. 中成药治疗功能性消化不良临床应用指南(2021年)[J]. 中国中西医结合杂志, 2022, 42(1): 5-12. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZXJ202201001.htm
[6] 王佳, 吕冠华. 中医药治疗功能性消化不良临床研究概况[J]. 实用中医内科杂志, 2022, 36(4): 69-72.
[7] 张海萍, 陈慧勇, 骆建善. 半夏泻心汤加味联合吗丁啉辅助治疗功能性消化不良临床研究[J]. 新中医, 2019, 51(6): 59-61.
[8] 李军祥, 陈誩, 李岩. 功能性消化不良中西医结合诊疗共识意见(2017年)[J]. 中国中西医结合消化杂志, 2017, 25(12): 889-894.
[9] Masuy I, Van Oudenhove L, Tack J. Review article: treatment options for functional dyspepsia[J]. Aliment Pharmacol Ther, 2019, 49(9): 1134-1172. doi: 10.1111/apt.15191
[10] Shah A, Talley NJ, Holtmann G. Current and future approaches for diagnosing small intestinal dysbiosis in patients with symptoms of functional dyspepsia[J]. Front Neurosci, 2022, 16: 830356. doi: 10.3389/fnins.2022.830356
[11] Goyal O, Goyal P, Kishore H, et al. Quality of life in Indian patients with functional dyspepsia: translation and validation of the Hindi version of Short-Form Nepean Dyspepsia Index[J]. Indian J Gastroenterol, 2022, 41(4): 378-388. doi: 10.1007/s12664-021-01233-0
[12] Mounsey A, Barzin A, Rietz A. Functional dyspepsia: evaluation and management[J]. Am Fam Physician, 2020, 101(2): 84-88.
[13] Sayuk GS, Gyawali CP. Functional dyspepsia: diagnostic and therapeutic approaches[J]. Drugs, 2020, 80(13): 1319-1336. doi: 10.1007/s40265-020-01362-4
[14] Lacy BE, Cangemi DJ. Updates in functional dyspepsia and bloating[J]. Curr Opin Gastroenterol, 2022, 38(6): 613-619. doi: 10.1097/MOG.0000000000000882
[15] Black CJ, Paine PA, Agrawal A, et al. British Society of Gastroenterology guidelines on the management of functional dyspepsia[J]. Gut, 2022, 71(9): 1697-1723.
[16] Du L, Chen B, Kim JJ, et al. Micro-inflammation in functional dyspepsia: a systematic review and meta-analysis[J]. Neurogastroenterol Motil, 2018, 30(4): e13304.
[17] Tack J, Camilleri M. New developments in the treatment of gastroparesis and functional dyspepsia[J]. Curr Opin Pharmacol, 2018, 43: 111-117.
[18] Pesce M, Cargiolli M, Cassarano S, et al. Diet and functional dyspepsia: clinical correlates and therapeutic perspectives[J]. World J Gastroenterol, 2020, 26(5): 456-465.
[19] 王龙华, 王凤磊, 李静, 等. 功能性消化不良的中医辨治思路与对策[J]. 中华中医药杂志, 2021, 36(9): 5368-5371.
[20] 李淑兰, 陈爱娣. 中医辨证治疗脾胃气虚型功能性消化不良的临床研究[J]. 中医临床研究, 2021, 13(19): 118-120.
[21] 何文广, 刘小梨, 刘其龙, 等. 半夏泻心汤联合谷氨酰胺治疗化疗相关性腹泻及调节肠道菌群的实验研究[J]. 广东医学, 2023, 44(1): 25-32.
[22] 张迪, 李雨静, 吉静, 等. 半夏泻心汤调节胃电节律失常大鼠胃窦Cajal间质细胞自噬的作用[J]. 中国实验方剂学杂志, 2023, 29(6): 55-62.
[23] 赵志英, 杨志华, 霍曼, 等. 基于脑-肠轴理论探讨小儿厌食的发病机制[J]. 中医临床研究, 2022, 14(4): 60-62.
[24] 邢相宜, 晏子俊, 段磊, 等. 六味能消胶囊联合复方消化酶对功能性消化不良患者临床症状及脑-肠轴相关因子水平的影响[J]. 现代生物医学进展, 2021, 21(9): 1702-1705.
[25] 邹毅成, 彭桑, 邱小蕾, 等. 功能性消化不良脑-肠轴神经递质与临床症状关联研究[J]. 湖北科技学院学报(医学版), 2021, 35(2): 116-119.
[26] 范明明, 张湘龙, 刘佳鑫, 等. 基于脑-肠轴理论探讨功能性消化不良的中医研究进展[J]. 中南大学学报(医学版), 2019, 44(11): 1300-1305. https://www.cnki.com.cn/Article/CJFDTOTAL-HNYD201911017.htm
[27] 沈爱红, 张洪涛, 施有奎. 马来酸曲美布汀联合舒肝颗粒对功能性消化不良患者血清神经肽S受体-1、降钙素基因相关肽及胃动素的影响[J]. 中国药师, 2019, 22(8): 1493-1495, 1499. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYSG201908031.htm
[28] 张冲, 万荣, 杨云志, 等. 健胃消食口服液对功能性消化不良患儿血清神经肽Y水平、摄食情况的影响[J]. 临床消化病杂志, 2022, 34(6): 422-425.
[29] Guo Y, Wei W, Chen JD. Effects and mechanisms of acupuncture and electroacupuncture for functional dyspepsia: a systematic review[J]. World J Gastroenterol, 2020, 26(19): 2440-2457.
[30] 毛兰芳, 梁乾坤, 汪龙德, 等. 基于脑肠轴的疏肝健脾法促进功能性消化不良患者胃动力作用的研究[J]. 时珍国医国药, 2021, 32(1): 42-46. https://www.cnki.com.cn/Article/CJFDTOTAL-SZGY202101011.htm
-




 下载:
下载: