Effect of Huoxue Tongjiang formula on esophageal metaplasia and its effect on PGRN and TNF-α/TNFR1/NF-κB/CDX2 pathway in rats with Barrett esophagus
-
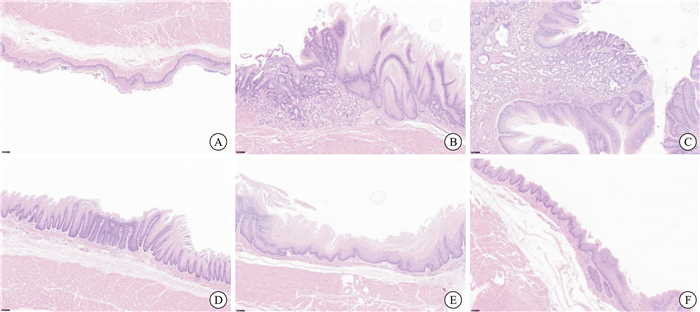
摘要: 目的 研究活血通降方对Barrett食管(Barrett esophagus,BE)模型大鼠PGRN及TNF-α/TNFR1/NF-κB/CDX2通路的影响,探讨其防治食管BE化生的作用机制。 方法 将SD大鼠随机分为对照组、模型组、西药组及中药高、中、低剂量组,每组10只。对照组行腹腔开关术,其余各组均采用“食管-十二指肠端侧吻合+保留全胃术联合铁剂注射”方法建立BE大鼠模型。造模4周后,各组分别给予相应剂量药物或相同体积生理盐水灌胃,2次/d,连续给药18周。苏木精-伊红染色评价食管黏膜病理表现;采用酶联免疫吸附法检测血清TNF-α、IL-8含量;采用蛋白免疫印记法检测食管组织中TNF-α、TNFR1、p-P65、CDX2、PGRN、GRN、MMP-12、SLPI和IL-8蛋白表达水平。 结果 对照组大鼠未发生BE化生及肠化(intestinal metaplasia,IM),模型组大鼠BE化生率为83.3%,明显高于中药治疗各组的50.0%、37.5%、28.6%以及西药组的57.1%,差异有统计学意义(P=0.023)。模型组出现IM的比例为66.7%,明显高于中药治疗各组的33.3%、12.5%、14.3%以及西药组的42.9%,差异有统计学意义(P=0.044)。其中,中药中、高剂量组BE化生率及IM发生率均最低。与对照组相比,模型组大鼠血清中TNF-α、IL-8的含量,食管组织中TNF-α、TNFR1、p-P65、CDX2、GRN、MMP-12和IL-8的表达水平均明显升高(P < 0.05),PGRN和SLPI的表达均显著下降(P < 0.05)。给予药物干预后,中药各剂量组大鼠TNF-α、IL-8含量,食管组织中TNF-α、TNFR1、p-P65、CDX2、GRN、MMP-12和IL-8的表达水平均显著降低(P < 0.05);PGRN和SLPI的表达均上调(P < 0.05)。其中,中药中、高剂量组血清中TNF-α、IL-8的含量及食管组织中GRN表达的降低均优于西药组(P < 0.05)。 结论 活血通降方可有效预防和治疗BE的化生及IM的发生,其作用机制可能与上调PGRN的表达及抑制炎症因子TNF-α、IL-8等的释放,进而抑制TNF-α/TNFR1/NF-κB/CDX2信号通路的活化有关。Abstract: Objective To explore the mechanism of the Huoxue Tongjiang formula in preventing and treating Barrett esophagus(BE) by investigating its effects on PGRN and the TNF-α/TNFR1/NF-κB/CDX2 pathway in rats with BE. Methods SD rats were randomly assigned to six groups: a control group, a model group, a Western medicine group, a high-dose traditional Chinese medicine group, a medium-dose traditional Chinese medicine group, and a low-dose traditional Chinese medicine group, with 10 rats in each group. The control group underwent laparotomy, whereas the other groups established BE rat models using the "esophago-duodenal end-to-side anastomosis combined with total gastrectomy and iron injection" method. Four weeks post-modeling, each group received the corresponding dose of medication or an equivalent volume of physiological saline via gavage, administered twice daily for a continuous duration of 18 weeks. HE staining was performed to evaluate the pathological changes in the esophageal mucosa. An enzyme-linked immunosorbent assay(ELISA) was utilized to detect serum levels of TNF-α and IL-8. Western blotting was employed to assess the expression levels of TNF-α, TNFR1, p-P65, CDX2, PGRN, GRN, MMP-12, SLPI, and IL-8 proteins in the esophageal tissues. Results No BE metaplasia or intestinal metaplasia(IM) was observed in the control group. In contrast, the BE metaplasia rate in the model group was 83.3%, significantly higher than the rates of 50.0%, 37.5%, and 28.6% in the traditional Chinese medicine treatment groups, as well as 57.1% in the Western medicine group(P=0.023). The incidence of IM in the model group was 66.7%, significantly higher than the incidences of 33.3%, 12.5%, and 14.3% in the traditional Chinese medicine treatment groups and 42.9% in the Western medicine group(P=0.044). Among these groups, the BE metaplasia rate and IM incidence were lowest in the medium and high-dose traditional Chinese medicine groups. Compared to the control group, serum levels of TNF-α and IL-8 in the model group, along with expression levels of TNF-α, TNFR1, p-P65, CDX2, GRN, MMP-12, and IL-8 in esophageal tissues, were significantly elevated(P < 0.05). In contrast, the expressions of PGRN and SLPI were significantly decreased(P < 0.05). Following drug intervention, levels of TNF-α and IL-8 in the serum of rats in each traditional Chinese medicine dosage group, along with expression levels of TNF-α, TNFR1, p-P65, CDX2, GRN, MMP-12, and IL-8 in esophageal tissues, were significantly reduced(P < 0.05). Conversely, the expressions of PGRN and SLPI were upregulated(P < 0.05). Among these groups, the medium and high-dose traditional Chinese medicine groups demonstrated superior effects on reducing serum TNF-α and IL-8 levels, as well as on the expression of GRN in esophageal tissues compared to the Western medicine group(P < 0.05). Conclusion The Huoxue Tongjiang formula effectively prevents and treats BE metaplasia and IM. Its mechanism may involve up-regulating the expression of PGRN and inhibiting the release of inflammatory factors TNF-α and IL-8, thereby inhibiting the activation of the TNF-α/TNFR1/NF-κB/CDX2 signaling pathway.
-
Key words:
- reflux esophagitis /
- Huoxue Tongjiang formula /
- intestinal metaplasia /
- CDX2 /
- progranulin
-

-
表 1 各组大鼠食管黏膜BE化生及IM的比较
例(%) 组别 例数 BE化生 IM 对照组 10 0 0 模型组 6 5(83.3) 4(66.7) 西药组 7 4(57.1) 3(42.9) 中药低剂量组 6 3(50.0) 2(33.3) 中药中剂量组 8 3(37.5) 1(12.5) 中药高剂量组 7 2(28.6) 1(14.3) χ2 12.990 11.400 P 0.023 0.044 表 2 各组大鼠食管组织中TNF-α、TNFR1、p-P65和CDX2蛋白表达的比较
X±S 组别 例数 TNF-α TNFR1 p-P65 CDX2 对照组 5 0.33±0.01 0.71±0.09 0.18±0.01 0.33±0.05 模型组 5 0.55±0.031) 0.15±0.051) 0.76±0.091) 0.45±0.051) 中药低剂量组 5 0.41±0.042) 0.71±0.072) 0.27±0.042) 0.35±0.032) 中药中剂量组 5 0.50±0.042) 0.67±0.082) 0.43±0.082) 0.39±0.042) 中药高剂量组 5 0.40±0.032) 0.72±0.042) 0.37±0.052) 0.34±0.052) 西药组 5 0.43±0.082) 0.84±0.062) 0.27±0.042) 0.36±0.042) 与对照组比较,1)P < 0.05;与模型组比较,2)P < 0.05。 表 3 各组大鼠食管组织中PGRN、GRN、MMP-12、SLPI和IL-8蛋白表达的比较
X±S 组别 例数 PGRN GRN MMP-12 SLPI IL-8 对照组 5 0.98±0.02 0.83±0.03 0.28±0.07 0.68±0.10 0.24±0.02 模型组 5 0.45±0.061) 1.32±0.041) 0.66±0.061) 0.24±0.051) 0.96±0.041) 中药低剂量组 5 0.71±0.032) 0.45±0.052)3) 0.47±0.072) 0.51±0.042) 0.35±0.042) 中药中剂量组 5 0.76±0.042) 0.38±0.062)3) 0.41±0.072) 0.48±0.062) 0.42±0.062) 中药高剂量组 5 0.78±0.052) 0.27±0.032)3) 0.36±0.042) 0.47±0.042) 0.29±0.072) 西药组 5 0.73±0.042) 0.87±0.052) 0.39±0.052) 0.36±0.072) 0.21±0.032) 与对照组比较,1)P < 0.05;与模型组比较,2)P < 0.05;与西药组比较,3)P < 0.05。 表 4 各组大鼠血清TNF-α和IL-8浓度的比较
pg/mL,X±S 组别 例数 IL-8 TNF-α 对照组 6 25.04±3.12 95.20±4.37 模型组 6 63.32±5.011) 158.30±3.651) 西药组 6 42.43±2.442) 132.20±5.182) 中药低剂量组 6 43.51±3.152) 130.21±4.252) 中药中剂量组 6 30.31±3.192)3) 105.32±3.692)3) 中药高剂量组 6 27.34±4.552)3) 100.10±4.532)3) 与对照组比较,1)P < 0.05;与模型组比较,2)P < 0.05;与西药组比较,3)P < 0.05。 -
[1] 中华医学会消化病学分会. 2020年中国胃食管反流病专家共识[J]. 中华消化杂志, 2020, 40(10): 649-663. doi: 10.3760/cma.j.cn311367-20200918-00558
[2] Mohy-Ud-Din N, Krill TS, Shah AR, et al. Barrett's esophagus: What do we need to know?[J]. Dis Mon, 2020, 66(1): 100850. doi: 10.1016/j.disamonth.2019.02.003
[3] 李鹏, 王拥军, 陈光勇, 等. 中国巴雷特食管及其早期腺癌筛查与诊治共识(2017, 万宁)[J]. 中华内科杂志, 2017, 56(9): 701-711.
[4] Klavan H, Russell MB, Macklin J, et al. Barrett's esophagus: A comprehensive review for the internist[J]. Dis Mon, 2018, 64(11): 471-487. doi: 10.1016/j.disamonth.2018.04.001
[5] Wang RH. From reflux esophagitis to Barrett's esophagus and esophageal adenocarcinoma[J]. World J Gastroenterol, 2015, 21(17): 5210-5219. doi: 10.3748/wjg.v21.i17.5210
[6] Bresalier RS. Chemoprevention of Barrett's Esophagus and Esophageal Adenocarcinoma[J]. Dig Dis Sci, 2018, 63(8): 2155-2162. doi: 10.1007/s10620-018-5149-6
[7] Alkhayyat M, Kumar P, Sanaka KO, et al. Chemoprevention in Barrett's esophagus and esophageal adenocarcinoma[J]. Therap Adv Gastroenterol, 2021, 14: 17562848211033730. doi: 10.1177/17562848211033730
[8] Fitzgerald RC, Abdalla S, Onwuegbusi BA, et al. Inflammatory gradient in Barrett's oesophagus: implications for disease complications[J]. Gut, 2002, 51: 316-322. doi: 10.1136/gut.51.3.316
[9] Garcia JM, Splenser AE, Kramer J, et al. Circulating inflammatory cytokines and adipokines are associated with increased risk of Barrett's esophagus: a case-control study[J]. Clin Gastroenterol Hepatol, 2014, 12: 229-238. e3. doi: 10.1016/j.cgh.2013.07.038
[10] Thrift AP, Garcia JM, El-Serag HB. A multibiomarker risk score helps predict risk for Barrett's esophagus[J]. Clin Gastroenterol Hepatol, 2014, 12: 1267-1271. doi: 10.1016/j.cgh.2013.12.014
[11] Beck F. The role of cdx genes in the mammalian gut[J]. Gut, 2004, 53(10): 1394-1396. doi: 10.1136/gut.2003.038240
[12] Li T, Guo H, Li H, et al. MicroRNA-92a-1-5p increases CDX2 by targeting FOXD1 in bile acids-induced gastric intestinal metaplasia[J]. Gut, 2019, 68(10): 1751-1763. doi: 10.1136/gutjnl-2017-315318
[13] Alquézar C, de la Encarnación A, Moreno F, et al. Progranulin deficiency induces overactivation of WNT5A expression via TNF-α/NF-κB pathway in peripheral cells from frontotemporal dementia-linked granulin mutation carriers[J]. J Psychiatry Neurosci, 2016, 41(4): 225-239. doi: 10.1503/jpn.150131
[14] Liu C, Li J, Shi W, et al. Progranulin Regulates Inflammation and Tumor[J]. Antiinflamm Antiallergy Agents Med Chem, 2020, 19(2): 88-102. doi: 10.2174/1871523018666190724124214
[15] Bateman A, Cheung ST, Bennett HPJ. A Brief Overview of Progranulin in Health and Disease[J]. Methods Mol Biol, 2018, 1806: 3-15.
[16] Horinokita I, Hayashi H, Oteki R, et al. Involvement of Progranulin and Granulin Expression in Inflammatory Responses after Cerebral Ischemia[J]. Int J Mol Sci, 2019, 20(20): 5210. doi: 10.3390/ijms20205210
[17] Hessman CL, Hildebrandt J, Shah A, et al. YB-1 Interferes with TNFα-TNFR Binding and Modulates Progranulin-Mediated Inhibition of TNFα Signaling[J]. Int J Mol Sci, 2020, 21(19): 7076. doi: 10.3390/ijms21197076
[18] 李培彩, 唐艳萍, 尹红, 等. 活血通降方对反流性食管炎大鼠食管内脏高敏感及LPS/TLR4/MC通路的影响[J]. 中国中西医结合消化杂志, 2023, 31(7): 538-544, 551. doi: 10.3969/j.issn.1671-038X.2023.07.11
[19] 尹红, 唐艳萍, 杨磊, 等. 活血通降方对反流性食管炎大鼠肠道菌群及Caspase-3/GSDME通路的影响[J]. 中国中西医结合消化杂志, 2022, 30(10): 701-707, 712. doi: 10.3969/j.issn.1671-038X.2022.10.04
[20] 刘琰, 唐艳萍, 刘磊, 等. 活血通降方对反流性食管炎模型大鼠食管动力、血清炎症因子及食管下括约肌SCF/c-kit信号通路的影响[J]. 中医杂志, 2022, 63(3): 269-275.
[21] 刘磊, 唐艳萍, 弓艳霞, 等. 活血通降方对反流性食管炎大鼠食管黏膜NF-κB/MAPK信号转导通路的影响[J]. 中国中西医结合外科杂志, 2021, 27(4): 555-562.
[22] Liu Y, Tang YP, Li PC, et al. Huoxue Tongjiang decoction-resisted reflux esophagitis by activation stem cell factor/c-kit/interstitial cell of cajal pathway and regulating the T-helper 17/regulatory T-cells balance in rats[J]. TMR, 2024, 9(12): 68.
[23] Buttar NS, Wang KK, Leontovich O, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus[J]. Gastroenterology, 2002, 122(4): 1101-1112.
[24] Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study[J]. J Natl Cancer Inst, 2011, 103: 1049-1057.
[25] Westra WM, Straub D, Milano F, et al. Inhibition of the BMP pathway prevents development of Barrett's-associated adenocarcinoma in a surgical rat model[J]. Dis Esophagus, 2022, 35(5): doab072.
[26] Kohata Y, Nakahara K, Tanigawa T, et al. Rebamipide Alters the Esophageal Microbiome and Reduces the Incidence of Barrett's Esophagus in a Rat Model[J]. Dig Dis Sci, 2015, 60(9): 2654-2661.
[27] 房渝, 任冬仁, 陈浩, 等. Barrett食管和食管腺癌的动物模型[J]. 胃肠病学, 2012, 17(4): 193-197.
[28] Shaheen NJ, Falk GW, Iyer PG, et al. American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus[J]. Am J Gastroenterol, 2016, 111(1): 30-50.
[29] 黄川锋, 刘安丽, 贺生, 等. TNF-α、COX-2、P53及CK7在Barrett食管中的表达及其意义分析[J]. 中国实用医药, 2017, 12(20): 3-6.
[30] Souza RF. Reflux esophagitis and its role in the pathogenesis of Barrett's metaplasia[J]. J Gastroenterol, 2017, 52(7): 767-776.
-

计量
- 文章访问数: 172
- 施引文献: 0



 下载:
下载:
