The therapeutic intervention of Chinese herbal formula SQQT on gastric mucosal spasmolytic polypeptide-expressing metaplasia cells and its systematic biological mechanism analysis
-
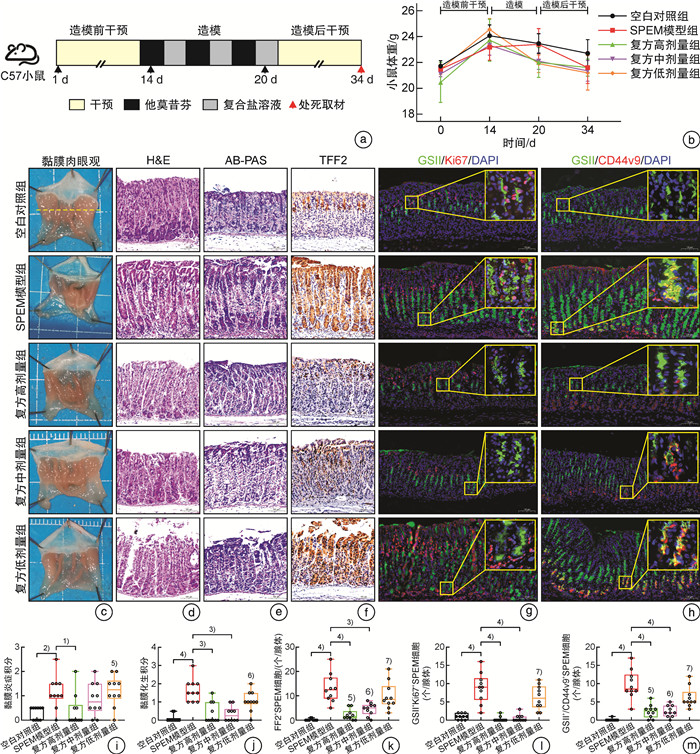
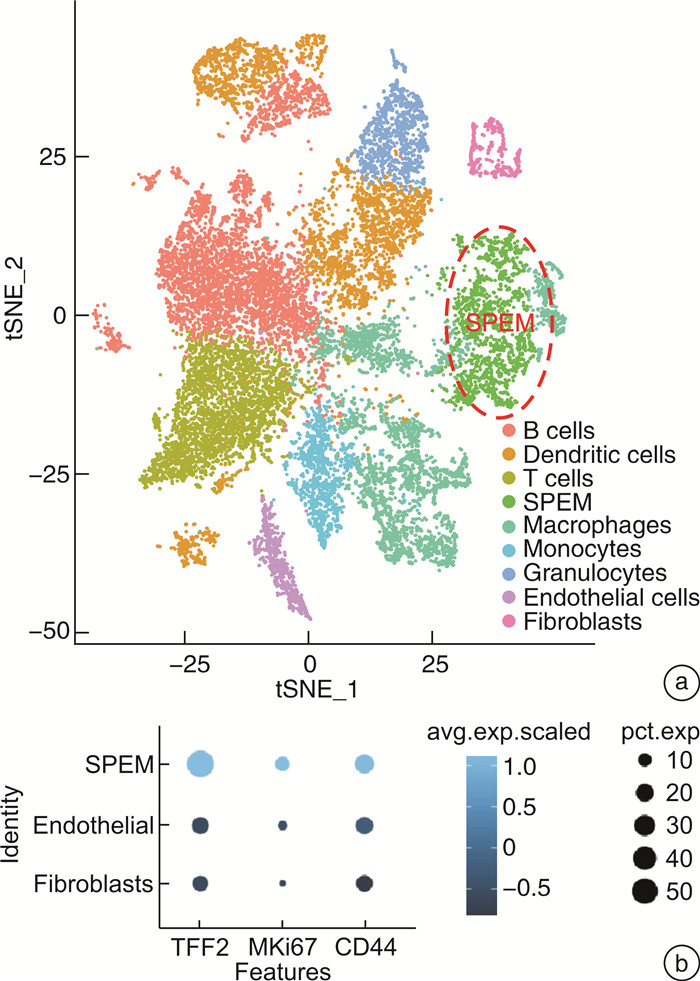
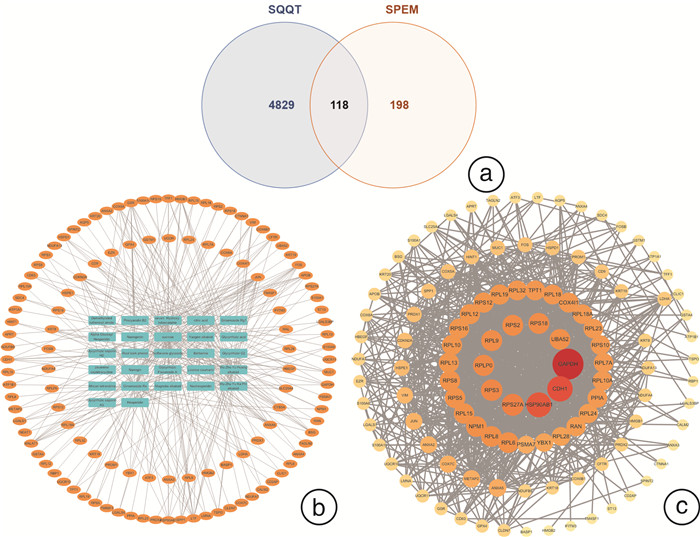
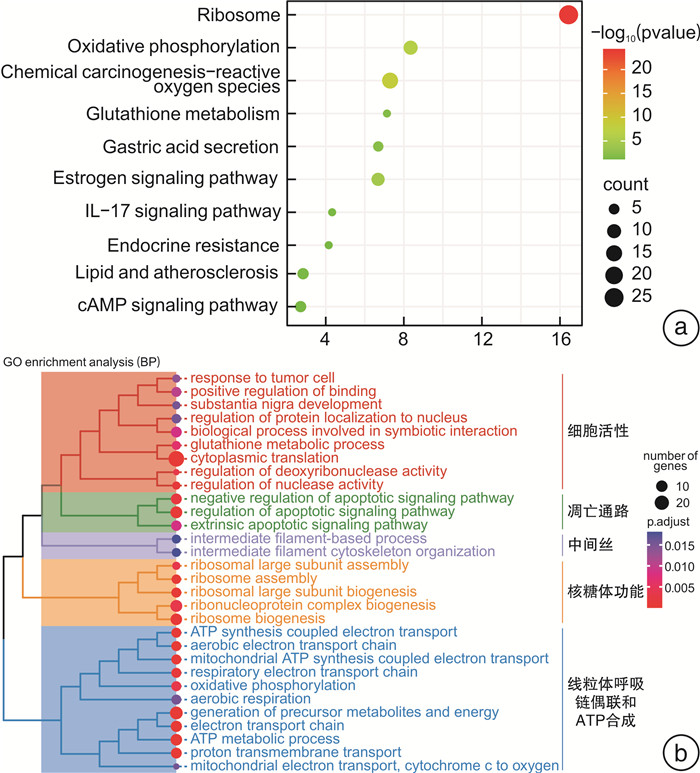
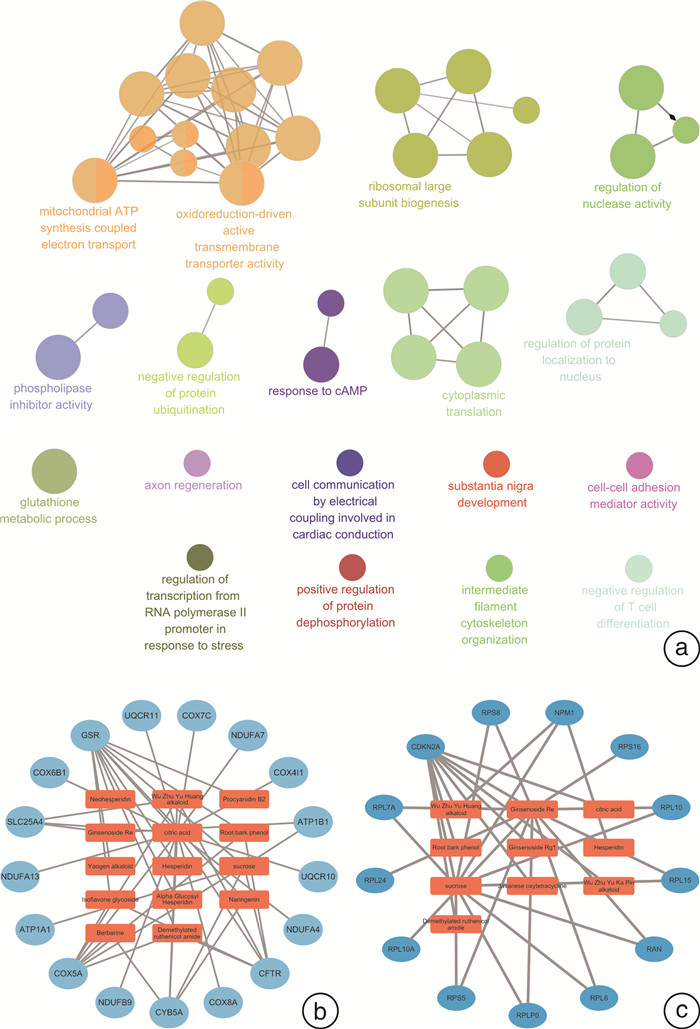
摘要: 目的 研究中药复方SQQT对胃黏膜解痉多肽表达化生(SPEM)细胞病理表型的影响,探讨其干预SPEM细胞病理演变的可能效应机制。方法 回顾性分析以SQQT为主方的中药辨证方对临床不同部位胃黏膜癌前病变的干预效果和SPEM细胞分布情况;评估SQQT对小鼠模型SPEM细胞病理表型的影响;利用超高效液相色谱、单细胞RNA转录数据挖掘和网络药理学分析等系统生物学方法构建“成分-靶点”网络图谱,并进行拓扑分析。结果 ①中药辨证方可有效减轻胃癌前病变患者胃体黏膜萎缩、肠化、异型增生的程度,抑制胃体黏膜基底部SPEM细胞的形成;②SQQT可显著降低他莫昔芬复合法诱导的增殖型SPEM小鼠胃体黏膜SPEM细胞数量,并有效抑制SPEM细胞Ki67和CD44v9标记蛋白的异常表达;③本研究从复方中共鉴定出27种成分和118个靶点与增殖型SPEM细胞的病理转变密切关联。拓扑分析显示,SQQT主要通过调控细胞能量代谢、核糖体功能、细胞通讯、凋亡信号通路等,影响SPEM细胞的增殖和分化功能。有6种化学成分(柠檬酸、根皮酚、去甲基吴茱萸酰胺、吴茱萸黄碱、橙皮苷和人参皂苷Re)同时参与调节SPEM细胞的线粒体ATP合成电子转运、氧化还原膜转运体活性以及核糖体功能,可能是SQQT调控SPEM细胞病理演变的潜在物质基础。结论 中药复方SQQT通过多种成分对应多个靶点的整体调控作用,实现抑制胃黏膜SPEM细胞异常增殖和分化。Abstract: Objective This study aimed to investigate the therapeutic impact of SQQT on the pathological phenotype of spasmolytic polypeptide-expressing metaplasia (SPEM) cells in the gastric mucosa, and to explore the potential mechanisms underlying its intervention in the pathological evolution of SPEM cells.Methods Retrospective analysis was conducted to assess SQQT's effect on gastric precancerous lesions and SPEM cell distribution. In a murine model, the effect of SQQT on SPEM cytopathic phenotype was evaluated. A "component-target" network map was constructed using chromatography, single-cell RNA data, and network pharmacology analysis, followed by topological analysis.Results ① SQQT effectively alleviated gastric mucosal atrophy, intestinal metaplasia, and dysplasia in patients with precancerous gastric lesions, and reduced SPEM cell formation at the gastric mucosal base; ②Notably, SQQT significantly reduced the count of SPEM cells in the gastric mucosa of mice induced by the tamoxifen compound method, effectively targeting and suppressing the abnormal expression of key marker proteins such as Ki67 and CD44v9. ③ This study identified a total of 27 components and 118 targets from the compound that are intimately associated with the pathological transition of proliferating SPEM cells. Topological analysis revealed that SQQT primarily regulates cellular processes including energy metabolism, ribosomal function, cell communication, and apoptotic signaling pathways, thereby exerting a profound impact on SPEM cell proliferation and differentiation. Intriguingly, six specific chemical constituents—Citric acid, Root bark phenol, Demethylated ruthenic amide, Wu Zhu Yu Huang alkaloid, Hesperidin, and Ginsenoside Re—were implicated in regulating critical biological functions such as mitochondrial ATP synthesis electron transport, redox membrane transporter activity, and ribosome function in SPEM cells, potentially constituting the material basis for compound regulation of SPEM cell pathology.Conclusion The traditional Chinese medicine compound SQQT effectively suppresses abnormal proliferation and differentiation of SPEM cells in the gastric mucosa by harnessing the synergistic effects of multiple components targeting diverse biological pathways.
-

-
表 1 实验分组和药物干预信息表
组别 剂量和浓度 干预方式 空白对照组 蒸馏水0.1 mL/10 g/d 灌胃 SPEM模型组 蒸馏水0.1 mL/10 g/d 灌胃 复方高剂量组 1.84 g/mL/d 灌胃 复方中剂量组 0.92 g/mL/d 灌胃 复方低剂量组 0.46 g/mL/d 灌胃 表 2 小鼠胃组织病理学评分
病理改变 分数 1 2 3 4 黏膜炎症
(H&E)黏膜及黏膜下层有斑块浸润的混合白细胞,显著存在粒细胞浸润则额外计分0.5。 多灶性联合白细胞浸润未扩展至黏膜下层 淋巴滤泡中白细胞数量显著增加,延伸至肌层 全黏膜层炎症 黏膜化生
(AB-PAS)胃体罕见小病灶 影响范围<1/3腺体细胞的中等病灶 影响范围在1/3~2/3腺体细胞的大病灶 影响范围>2/3腺体细胞的大病灶 表 3 不同部位胃黏膜病理组织学疗效等级分析
例(%) 病变 叶酸组 中药辨证方组 OR 95%CI P 例数 有效 例数 有效 炎症 胃体 32 12(37.5) 68 21(30.9) 1.343 0.556~3.242 0.637 胃窦 97 28(28.9) 190 60(31.6) 0.879 0.515~1.502 0.512 总体 129 40(31.0) 259 81(31.3) 0.984 0.623~1.553 0.944 萎缩 胃体 26 15(57.7) 38 27(71.1) 0.556 0.195~1.583 0.166 胃窦 82 38(46.3) 167 93(44.3) 0.687 0.404~1.168 0.271 总体 108 53(49.1) 205 120(58.5) 0.658 0.401~1.056 0.083 肠化 胃体 20 10(50.0) 29 17(58.6) 0.706 0.224~2.221 0.891 胃窦 75 37(49.3) 153 74(48.4) 1.039 0.598~1.807 0.552 总体 95 47(49.5) 182 91(50.0) 0.966 0.587~1.590 0.892 异型增生 胃体 10 7(70.0) 18 14(77.8) 0.667 0.116~3.838 0.821 胃窦 60 41(68.3) 126 84(66.7) 1.079 0.559~2.084 0.650 总体 70 48(68.6) 144 98(68.1) 1.018 0.550~1.883 0.956 -
[1] He Y, Wang Y, Luan F, et al. Chinese and global burdens of gastric cancer from 1990 to 2019[J]. Cancer Med, 2021, 10(10): 3461-3473. doi: 10.1002/cam4.3892
[2] Sáenz JB, Mills JC. Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer[J]. Nat Rev Gastroenterol Hepatol, 2018, 15(5): 257-273. doi: 10.1038/nrgastro.2018.5
[3] Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma[J]. Lab Invest, 1999, 79(6): 639-646.
[4] Halldórsdóttir AM, Sigurdardóttrir M, Jónasson JG, et al. Spasmolytic polypeptide-expressing metaplasia (SPEM) associated with gastric cancer in Iceland[J]. Dig Dis Sci, 2003, 48(3): 431-441. doi: 10.1023/A:1022564027468
[5] Yamaguchi H, Goldenring JR, Kaminishi M, et al. Identification of spasmolytic polypeptide expressing metaplasia (SPEM) in remnant gastric cancer and surveillance postgastrectomy biopsies[J]. Dig Dis Sci, 2002, 47(3): 573-578. doi: 10.1023/A:1017920220149
[6] 王萍, 尹晓岚, 张北华, 等. 近40年慢性萎缩性胃炎及胃癌前病变中医研究述评[J]. 中医杂志, 2020, 61(22): 1943-1947. https://www.cnki.com.cn/Article/CJFDTOTAL-ZZYZ202022006.htm
[7] Zhang T, Zhang B, Xu J, et al. Chinese herbal compound prescriptions combined with Chinese medicine powder based on traditional Chinese medicine syndrome differentiation for treatment of chronic atrophic gastritis with erosion: a multi-center, randomized, positive-controlled clinical trial[J]. Chin Med, 2022, 17(1): 142. doi: 10.1186/s13020-022-00692-7
[8] 中华医学会消化病学分会, 中华医学会消化病学分会消化系统肿瘤协作组. 中国慢性胃炎诊治指南(2022年, 上海)[J]. 胃肠病学, 2023, 28(3): 149-180. https://xuewen.cnki.net/CCND-JFRB202402240013.html
[9] 中华医学会病理分会消化病理学组筹备组. 慢性胃炎及上皮性肿瘤胃黏膜活检病理诊断共识[J]. 中华病理学杂志, 2017, 46(5): 289-293. doi: 10.3760/cma.j.issn.0529-5807.2017.05.001
[10] Saenz JB, Burclaff J, Mills JC. Modeling Murine Gastric Metaplasia Through Tamoxifen-Induced Acute Parietal Cell Loss[J]. Methods Mol Biol, 2016, 1422: 329-339.
[11] Huang Y, Chen S, Yao Y, et al. Ovotransferrin alleviated acute gastric mucosal injury in BALB/c mice caused by ethanol[J]. Food Funct, 2023, 14(1): 305-318. doi: 10.1039/D2FO02364D
[12] Chu F, Li Y, Meng X, et al. Gut Microbial Dysbiosis and Changes in Fecal Metabolic Phenotype in Precancerous Lesions of Gastric Cancer Induced With N-Methyl-N'-Nitro-N-Nitrosoguanidine, Sodium Salicylate, Ranitidine, and Irregular Diet[J]. Front Physiol, 2021, 12: 733979. doi: 10.3389/fphys.2021.733979
[13] Jin D, Huang K, Xu M, et al. Deoxycholic acid induces gastric intestinal metaplasia by activating STAT3 signaling and disturbing gastric bile acids metabolism and microbiota[J]. Gut Microbes, 2022, 14(1): 2120744. doi: 10.1080/19490976.2022.2120744
[14] Quilty F, Freeley M, Gargan S, et al. Deoxycholic acid induces proinflammatory cytokine production by model oesophageal cells via lipid rafts[J]. J Steroid Biochem Mol Biol, 2021, 214: 105987. doi: 10.1016/j.jsbmb.2021.105987
[15] Rogers AB, Taylor NS, Whary MT, et al. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice[J]. Cancer Res, 2005, 65(23): 10709-10715. doi: 10.1158/0008-5472.CAN-05-1846
[16] Bockerstett KA, Petersen CP, Noto CN, et al. Interleukin 27 Protects From Gastric Atrophy and Metaplasia During Chronic Autoimmune Gastritis[J]. Cell Mol Gastroenterol Hepatol, 2020, 10(3): 561-579. doi: 10.1016/j.jcmgh.2020.04.014
[17] Burclaff J, Willet SG, Sáenz JB, et al. Proliferation and Differentiation of Gastric Mucous Neck and Chief Cells During Homeostasis and Injury-induced Metaplasia[J]. Gastroenterology, 2020, 158(3): 598-609.e5. doi: 10.1053/j.gastro.2019.09.037
[18] Xiong M, Chen X, Wang H, et al. Combining transcriptomics and network pharmacology to reveal the mechanism of Zuojin capsule improving spasmolytic polypeptide-expressing metaplasia[J]. J Ethnopharmacol, 2024, 318(Pt B): 117075.
[19] Chen WQ, Tian FL, Zhang JW, et al. Preventive and inhibitive effects of Yiwei Xiaoyu granules on the development and progression of spasmolytic polypeptide-expressing metaplasia lesions[J]. World J Gastrointest Oncol, 2021, 13(11): 1741-1754. doi: 10.4251/wjgo.v13.i11.1741
[20] Petersen CP, Weis VG, Nam KT, et al. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells[J]. Gastroenterology, 2014, 146(7): 1727-1738.e8. doi: 10.1053/j.gastro.2014.02.007
[21] Hong X, Li H, Lin Y, et al. Efficacy and potential therapeutic mechanism of Weiwei decoction on Spasmolytic polypeptide-expressing metaplasia in Helicobacter pylori-infected and Atp4a-knockout mice[J]. J Ethnopharmacol, 2024, 319(Pt 1): 117062.
[22] Teal E, Dua-Awereh M, Hirshorn ST, et al. Role of metaplasia during gastric regeneration[J]. Am J Physiol Cell Physiol, 2020, 319(6): C947-C954. doi: 10.1152/ajpcell.00415.2019
[23] Miao ZF, Sun JX, Adkins-Threats M, et al. DDIT4 Licenses Only Healthy Cells to Proliferate During Injury-induced Metaplasia[J]. Gastroenterology, 2021, 160(1): 260-271.e10. doi: 10.1053/j.gastro.2020.09.016
[24] Miao ZF, Cho CJ, Wang ZN, et al. Autophagy repurposes cells during paligenosis[J]. Autophagy, 2021, 17(2): 588-589. doi: 10.1080/15548627.2020.1857080
[25] Miao ZF, Adkins-Threats M, Burclaff JR, et al. A Metformin-Responsive Metabolic Pathway Controls Distinct Steps in Gastric Progenitor Fate Decisions and Maturation[J]. Cell Stem Cell, 2020, 26(6): 910-925.e6. doi: 10.1016/j.stem.2020.03.006
-





 下载:
下载: