Berberine as an add therapy for Helicobacter pylori infection: a randomized controlled trial
-
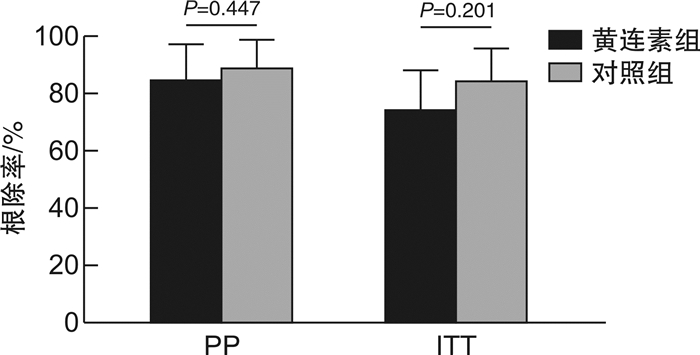
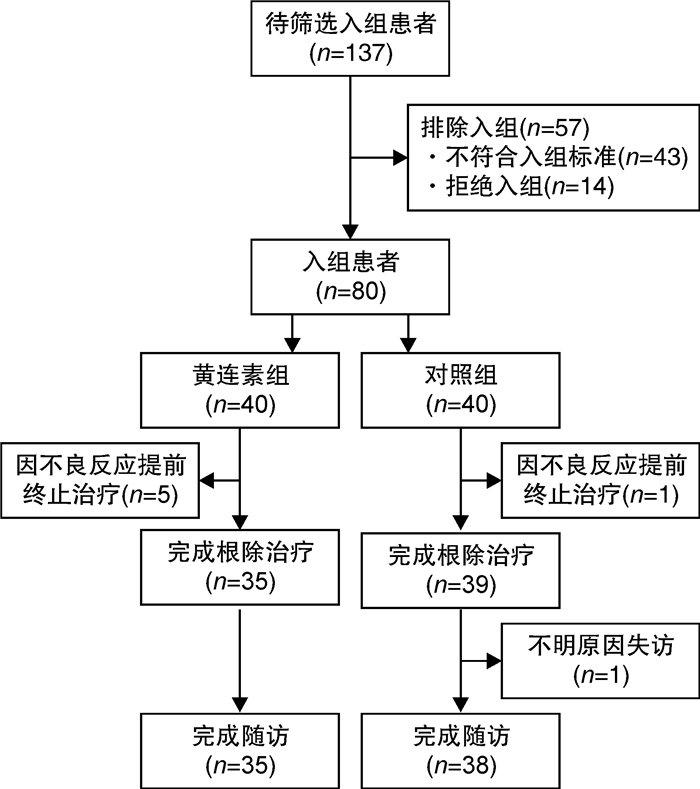
摘要: 目的 探究黄连素联合铋剂四联方案根除幽门螺杆菌(Helicobacter pylori,HP)感染的疗效和安全性。方法 本研究为随机、对照、开放标签临床试验研究,共纳入80例HP感染初治患者。入组患者随机分入黄连素组和对照组。对照组患者予以阿莫西林+克拉霉素+雷贝拉唑+枸橼酸铋钾方案治疗,黄连素组患者在对照组基础上加用黄连素治疗,两组治疗时间均为14 d。主要结局指标包括HP根除率和不良反应发生率。结果 黄连素组中5例患者因难以耐受的不良反应而提前终止治疗,对照组中1例患者因可疑药物相关皮疹而提前终止治疗,1例入组患者不明原因失访,最终73例患者完成了治疗和随访。在意向治疗分析(ITT)和方案分析(PP)中,黄连素组的根除率均低于对照组,但差异无统计学意义(PP:85.71% vs 89.47%,P=0.447;ITT:75.00% vs 85.00%,P=0.201)。黄连素组的总不良反应发生率显著高于对照组(74.29% vs 50.00%,P=0.029)。结论 黄连素联合BCQT治疗HP感染不能有效提高根除率,可能存在加重不良反应的风险。Abstract: Objective The present study aimed to assess the efficacy and safety of berberine combination in the bismuth containing quadruple therapy (BCQT) regimen as the first-line therapy for Helicobacter pylori(H.pylori) eradication.Methods This randomized, open-label, controlledtrial enrolled 80 patients with untreated H.pylori infection. Participants in the berberine group received eradication therapy with a rabeprazole-amoxicillin-clarithromycin-bismuth-berberine regimen for 14 days, whereas those in the control group received a rabeprazole-amoxicillin-clarithromycin-bismuth regimen for the same duration. The primary outcomes were eradication rate and side effects rate.Results Five participants in the berberine group dropped out due to intolerant side effects, while two participants in the control group opted out due to rash and loss to follow-up. In the intention-to-treat (ITT) and per-protocol (PP) analyses, the eradication rates in the berberine group were lower than those in the control group, without significant differences (PP: 85.71% vs 89.47%, P=0.447;ITT: 75.00% vs 85.00%, P=0.201). The total side-effect rate in the berberine group was higher than that in the control group (74.29% vs 50.00%, P=0.029).Conclusion We concluded that the combination of berberine and BCQT results in elevated and severe side effects. The hypothesis that berberine as an add-on therapy would improve eradication rate of BCQT was not confirmed.
-
Key words:
- berberine /
- Helicobacter pylori /
- eradication rate
-

-
表 1 HP耐药基因检测方法及突变位点
抗生素 基因 突变位点 检测方法 克拉霉素 23S rRNA A2143G,A2142G,A2142C DNA探针法 阿莫西林 PBP1 S414R,Y484C,T541I,P600T 测序法 左氧氟沙星 gyrA Asn87→Lys,Ala88→Val,Asp91→Gly 测序法 四环素 16S rRNA AGA→TTC 测序法 表 2 两组患者的基线资料比较
X±S,例(%) 基线资料 黄连素组
(n=40)对照组
(n=40)P 年龄/岁 37.80±11.88 41.13±11.52 0.691 性别 0.369 男 20(50.00) 16(40.00) 女 20(50.00) 24(60.00) 吸烟史 7(17.50) 11(27.50) 0.312 饮酒史 15(37.50) 18(45.00) 0.496 慢性萎缩性胃炎 9(22.50) 15(37.50) 0.143 阿莫西林耐药 0 2(5.00) 0.268 左氧氟沙星耐药 10(25.00) 13(32.50) 0.385 克拉霉素耐药 14(35.00) 24(60.00) 0.025 甲硝唑耐药 39(97.50) 39(97.50) 1.000 四环素耐药 2(5.00) 6(15.00) 0.136 表 3 两组患者HP根除率比较
例 根除情况 黄连素组
(n=40)对照组
(n=40)P 根除情况 根除成功 30 34 根除失败 5 4 脱落、失访 5 2 PP分析 85.71%,
95%CI:
97.30~74.1489.47%,
95%CI:
79.71~99.230.447 ITT分析 75.00%,
95%CI:
61.58~88.4285.00%,
95%CI:
73.93~96.070.201 表 4 两组患者药物不良反应情况比较
例(%) 不良反应 黄连素组
(n=35)对照组
(n=38)P 恶心 12(34.29) 5(13.16) 0.031 呕吐 3(8.57) 1(2.63) 0.277 腹痛 1(2.86) 1(2.63) 0.732 腹胀 1(2.86) 1(2.63) 0.732 眩晕 4(11.43) 5(13.16) 0.553 腹泻 11(31.43) 9(23.68) 0.289 睡眠障碍 2(5.71) 0 0.226 味觉障碍 12(34.29) 11(28.95) 0.406 合计 26(74.29) 19(50.00) 0.029 -
[1] Ding SZ, Du YQ, Lu H, et al. Chinese Consensus Report on Family-Based Helicobacter pylori Infection Control and Management(2021 Edition)[J]. Gut, 2022, 71(2): 238-253. doi: 10.1136/gutjnl-2021-325630
[2] Iwamuro M, Kusumoto C, Nakagawa M, et al. Endoscopic features of oxyntic gland adenoma and gastric adenocarcinoma of the fundic gland type differ between patients with and without Helicobacter pylori infection: a retrospective observational study[J]. BMC Gastroenterol, 2022, 22(1): 294. doi: 10.1186/s12876-022-02368-w
[3] Liou JM, Malfertheiner P, Lee YC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus[J]. Gut, 2020, 69(12): 2093-2112. doi: 10.1136/gutjnl-2020-322368
[4] Oh CK, Lim H, Seo SI, et al. Efficacy comparison of 7-and 14-day P-CAB based bismuth-containing quadruple regimen with PPI based bismuth-containing quadruple regimen for Helicobacter pylori infection: rationale and design of an open-label, multicenter, randomized controlled trial[J]. BMC Gastroenterol, 2023, 23(1): 453. doi: 10.1186/s12876-023-03100-y
[5] Chtourou L, Moalla M, Mnif B, et al. Prevalence of Helicobacter pylori resistance to clarithromycin in Tunisia[J]. J Med Microbiol, 2022, 71(8).
[6] Ayinde O, Ross JD. The frequency and duration of side-effects associated with the use of oral metronidazole; A prospective study of VITA trial participants[J]. Int J STD AIDS, 2023, 34(12): 897-902. doi: 10.1177/09564624231179505
[7] Li J, Shi H, Zhou F, et al. The Efficacy and Safety of Regimens for Helicobacter pylori Eradication Treatment in China: A Systemic Review and Network Meta-Analysis[J]. J Clin Gastroenterol, 2024, 58(1): 12-23. doi: 10.1097/MCG.0000000000001902
[8] Yu H, Zhang S, Li R, et al. Berberine alleviates inflammation and suppresses PLA2-COX-2-PGE2-EP2 pathway through targeting gut microbiota in DSS-induced ulcerative colitis[J]. Biochem Biophys Res Commun, 2024, 695: 149411. doi: 10.1016/j.bbrc.2023.149411
[9] Wang Y, Zhao D, Su L, et al. Therapeutic potential of berberine in attenuating cholestatic liver injury: insights from a PSC mouse model[J]. Cell Biosci, 2024, 14(1): 14. doi: 10.1186/s13578-024-01195-8
[10] Lai R, Lin Z, Yang C, et al. Novel berberine derivatives as p300 histone acetyltransferase inhibitors in combination treatment for breast cancer[J]. Eur J Med Chem, 2024, 266: 116116. doi: 10.1016/j.ejmech.2023.116116
[11] Pavlova JA, Tereshchenkov AG, Nazarov PA, et al. Conjugates of chloramphenicol amine and berberine as antimicrobial agents[J]. Antibiotics(Basel), 2022, 12(1): 15.
[12] Di Somma A, Cane C, Rotondo NP, et al. A comparative study of the inhibitory action of berberine derivatives on the recombinant protein FtsZ of E. coli[J]. Int J Mol Sci, 2023, 24(6): 5674. doi: 10.3390/ijms24065674
[13] Lin YH, Lin JH, Chou SC, et al. Berberine-loaded targeted nanoparticles as specific Helicobacter pylori eradication therapy: in vitro and in vivo study[J]. Nanomedicine(London, England), 2015, 10(1): 57-71. doi: 10.2217/nnm.14.76
[14] Mahady GB, Pendland SL, Stoia A, et al. In vitro susceptibility of Helicobacter pylori to isoquinoline alkaloids from Sanguinaria canadensis and Hydrastis canadensis[J]. Phytother Res, 2003, 17(3): 217-221. doi: 10.1002/ptr.1108
[15] Huang YQ, Huang GR, Wu MH, et al. Inhibitory effects of emodin, baicalin, schizandrin and berberine on hefA gene: treatment of Helicobacter pylori-induced multidrug resistance[J]. World J Gastroenterol, 2015, 21(14): 4225-4231. doi: 10.3748/wjg.v21.i14.4225
[16] Jiang X, Jiang C, Huang C, et al. Berberine Combined with Triple Therapy versus Triple Therapy for Helicobacter pylori Eradication: A Meta-Analysis of Randomized Controlled Trials[J]. Evid Based Complement Alternat Med, 2018, 2018: 8716910.
[17] Gao C, Du SY, Fang L, et al. Eradication treatment of Helicobacter pylori infection based on molecular pathologic antibiotic resistance[J]. Infect Drug Resist, 2020, 13: 69-79. doi: 10.2147/IDR.S232169
[18] Yang T, Wang R, Liu H, et al. Berberine regulates macrophage polarization through IL-4-STAT6 signaling pathway in Helicobacter pylori-induced chronic atrophic gastritis[J]. Life Sci, 2021, 266: 118903. doi: 10.1016/j.lfs.2020.118903
[19] Zhang D, Ke L, Ni Z, et al. Berberine containing quadruple therapy for initial Helicobacter pylori eradication: An open-label randomized phase Ⅳ trial[J]. Medicine, 2017, 96(32): e7697. doi: 10.1097/MD.0000000000007697
[20] Sharma NR, Wagle A, Bist M, et al. Clarithromycin-induced acute liver injury in a patient with positive Helicobacter pylori: a case report and review of the literature[J]. Ann Med Surg(Lond), 2023, 85(9): 4629-4632. doi: 10.1097/MS9.0000000000001135
[21] Hansen MP, Scott AM, McCullough A, et al. Adverse events in people taking macrolide antibiotics versus placebo for any indication[J]. Cochrane Database Syst Rev, 2019, 1(1): CD011825.
[22] Ford AC, Malfertheiner P, Giguere M, et al. Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis[J]. World J Gastroenterol, 2008, 14(48): 7361-7370. doi: 10.3748/wjg.14.7361
[23] Blais JE, Huang X, Zhao JV. Overall and Sex-Specific Effect of Berberine for the Treatment of Dyslipidemia in Adults: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials[J]. Drugs, 2023, 83(5): 403-427. doi: 10.1007/s40265-023-01841-4
[24] Aksoy EK, Sapmaz FP, Goktas Z, et al. Comparison of Helicobacter pylori eradication rates of 2-week levofloxacin-containing triple therapy, levofloxacin-containing bismuth quadruple therapy, and standard bismuth quadruple therapy as a first-line regimen[J]. Med Princ Pract, 2017, 26(6): 523-529. doi: 10.1159/000484930
[25] Chen C, Yu Z, Li Y, et al. Effects of berberine in the gastrointestinal tract-a review of actions and therapeutic implications[J]. Am J Chin Med, 2014, 42(5): 1053-1070. doi: 10.1142/S0192415X14500669
[26] Xin HW, Wu XC, Li Q, et al. The effects of berberine on the pharmacokinetics of cyclosporin A in healthy volunteers[J]. Methods Find Exp Clin Pharmacol, 2006, 28(1): 25-29. doi: 10.1358/mf.2006.28.1.962774
[27] Chatterjee P, Franklin MR. Human cytochrome p450 inhibition and metabolic-intermediate complex formation by goldenseal extract and its methylenedioxyphenyl components[J]. Drug Metab Dispos, 2003, 31(11): 1391-1397. doi: 10.1124/dmd.31.11.1391
[28] Jenkins H, Jenkins R, Patat A. Effect of Multiple Oral Doses of the Potent CYP3A4 Inhibitor Clarithromycin on the Pharmacokinetics of a Single Oral Dose of Vonoprazan: A PhaseⅠ, Open-Label, Sequential Design Study[J]. Clin Drug Investig, 2017, 37(3): 311-316. doi: 10.1007/s40261-016-0488-6
-




 下载:
下载: