The regulation of dietary lamb on the macrophage polarization in mice with ulcerative colitis
-
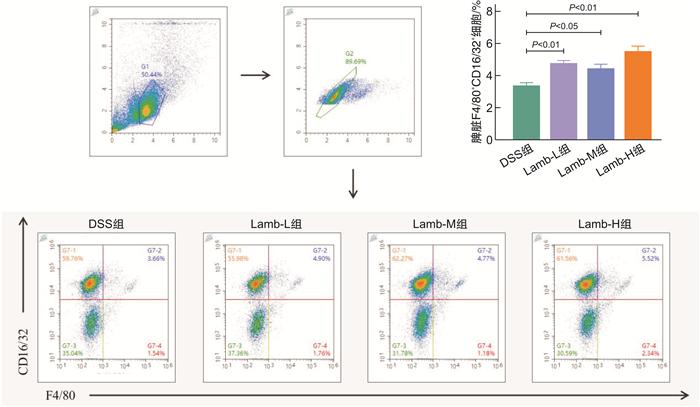
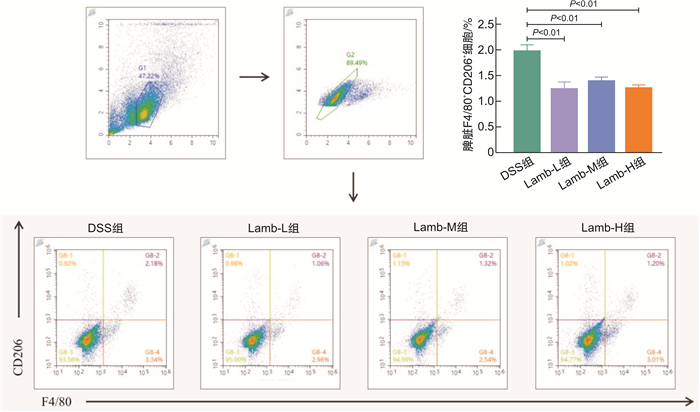
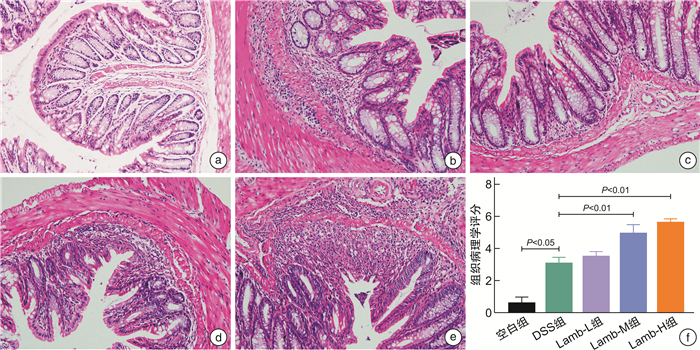
摘要: 目的 研究饮食“发物”羊肉对溃疡性结肠炎(ulcerative colitis,UC)小鼠的影响,为UC患者的饮食方案提供理论依据。方法 将32只SPF级健康雌性C57BL/6小鼠随机分为空白组、模型组、羊肉低剂量组、羊肉中剂量组、羊肉高剂量组。空白组小鼠全程自由进食饮水,模型组和羊肉低、中、高各剂量组均自由饮用2.5 %(w/v)葡聚糖硫酸钠(DSS)溶液5 d、去离子水7 d为1个周期,重复3个周期以建立慢性UC模型,以开始给予DSS溶液为第1天,第6天开始给予羊肉各剂量组0.25、0.50、1.00 g/mL浓度的羊肉混悬液灌胃,模型组给予等量去离子水灌胃,期间每日观察小鼠一般情况,测量体重、检测便潜血、记录粪便性状,计算疾病活动指数(disease activity index,DAI)。干预结束后取结肠组织,苏木精-伊红染色评估病理损伤程度并进行组织病理学评分;取脾脏组织,制备单细胞悬液,流式细胞术检测M1型、M2型巨噬细胞的水平;RT-qPCR检测结肠黏膜紧密连接相关蛋白(ZO-1、E-cadherin)、M1型巨噬细胞因子(TNF-α、IL-6)、M2型巨噬细胞因子(IL-10、TGF-β)的mRNA含量。结果 与空白组比较,UC小鼠均表现出不同程度的体重下降、大便稀溏、便血;与模型组比较,羊肉各剂量组小鼠体重明显下降(P<0.01或P<0.05),DAI呈升高趋势,其中中、高剂量组差异有统计学意义(P<0.05);与模型组比较,羊肉各剂量组小鼠结肠病理损伤程度呈升高趋势,其中中、高剂量组差异有统计学意义(P<0.01);羊肉各剂量组的结肠ZO-1、E-cadherin mRNA呈降低趋势,其中中、高剂量组差异有统计学意义(P<0.01);与模型组比较,羊肉各剂量组小鼠脾脏M1型巨噬细胞表达升高(P<0.01或P<0.05),相关细胞因子TNF-α、IL-6 mRNA水平升高,其中高剂量组差异有统计学意义(P<0.01),脾脏M2型巨噬细胞表达降低(P<0.01),相关细胞因子IL-10、TGF-β mRNA水平降低(P<0.01或P<0.05)。结论 饮食“发物”羊肉会破坏小鼠UC结肠黏膜屏障,加重肠道炎症表现,其机制可能与促进巨噬细胞向M1细胞分化、抑制向M2细胞分化,从而加重M1/M2型巨噬细胞失衡有关。Abstract: Objective To study the effect of lamb on macrophage polarization in mice with ulcerative colitis(UC) induced by dextran sodium sulfate(DSS) and provide a theoretical basis for the dietary regimen of UC patients.Methods Thirty-two healthy female C57BL/6 mice with SPF grade were randomly divided into control group, DSS group, low-dose lamb group, medium-dose group and high-dose lamb group. The mice in the control group ate and drank freely throughout the process. The DSS group and the low, medium and high dose group of lamb were free to drink 2.5%(w/v) DSS solution for 5 days and deionized water for 7 days for 1 cycle, repeated 3 cycles to establish a chronic UC model. Take the start of giving DSS solution as day 1, started to gavage lamb suspension of 0.25 g/mL, 0.50 g/mL, 1.00 g/mL dose on day 6. The DSS group was given the same amount of deionized water, during which the general condition, weight, and stool of all groups were recorded every day. Colon tissue was retained after the intervention and the degree of pathological damage was assessed by H & E staining and histopathological scoring evaluation. Spleen tissue was collected to prepare single-cell suspension and the level of M1 and M2 macrophages was measured by flow cytometry and the content of TNF-α, IL-6, IL-10, TGF-β, ZO-1, E-cadherin mRNA were detected by RT-qPCR.Results Compared with the control group, UC mice showed different degrees of weight loss, diarrhea and bloody stool. Compared with the DSS group, the weight of mice in each dose group of lamb decreased significantly(P < 0.01 or P < 0.05) and DAI showed an increasing trend, among which there were statistical differences in the middle and high-dose group(P < 0.05). Compared with the DSS group, the degree of pathological damage of the colon of mice in each dose group of lamb showed an increasing trend, and there were statistical differences in the middle and high dose group(P < 0.01). The colonic tight junction-associated proteins ZO-1 and E-cadherin mRNA in each dose group of lamb showed a decreasing trend and there were differences in the medium and high dose group(P < 0.01). Compared with the DSS group, the content of M1 macrophages in the spleen of mice in each dose group of lamb was significantly increased(P < 0.01 or P < 0.05). The content of M1 macrophage-associated inflammatory factors TNF-α and IL-6 mRNA showed an increasing trend and there were statistical differences in the high-dose group(P < 0.01). The content of M2 macrophages was significantly reduced(P < 0.01) and the content of M2 macrophages-associated inflammatory factors IL-10 and TGF-β mRNA decreased(P < 0.01 or P < 0.05).Conclusion Dietary lamb can damage the mucosal barrier and aggravate the intestinal inflammation of UC and its mechanism is related to promoting the differentiation of macrophages to M1 cells and inhibiting the differentiation to M2 cells, thereby aggravating the imbalance of M1/M2 macrophages.
-
Key words:
- ulcerative colitis /
- lamb /
- macrophage /
- immune regulation /
- intestinal inflammation
-

-
表 1 DAI评分标准
指标 评分/分 0 1 2 3 4 体重下降指数 无下降 1%~5% >5%~10% >10%~15% >15% 粪便性状 正常 松散 腹泻 粪便潜血 无 阳性 肉眼血便 表 2 结肠组织病理学评分标准
组织学改变 评分/分 0 1 2 3 炎症细胞浸润 无 轻微 中等 严重 组织损伤深度 无 黏膜层 黏膜下层 黏膜肌层 表 3 本实验所用引物
基因 上游(5'-3') 下游(5'-3') 扩增片段长度/bp GAPDH TGGAATCCTGTGGCATCCATGAAAC TAAAACGCAGCTCAGTAACAGTCCG 349 IL-6 TAGTCCTTCCTACCCCAATTTCC TTGGTCCTTAGCCACTCCTTC 76 IL-10 CCACAAAGCAGCCTTGCA AGTAAGAGCAGGCAGCATAGCA 73 TNF-α CCCTCACACTCAGATCATCTTCT GCTACGACGTGGGCTACAG 61 TGF-β CTCCCGTGGCTTCTAGTGC GCCTTAGTTTGGACAGGATCTG 133 ZO-1 CTTCTCTTGCTGGCCCTAAAC TGGCTTCACTTGAGGTTTCTG 101 E-cadherin CGACCGGAAGTGACTCGAAAT TCAGAACCACTGCCCTCGTAAT 188 表 4 各组小鼠结肠ZO-1、E-cadherin mRNA含量的比较
X±S 组别 例数 ZO-1 mRNA E-cadherin mRNA DSS组 6 1.028±0.275 1.016±0.161 Lamb-L组 6 0.785±0.173 0.635±0.351 Lamb-M组 7 0.274±0.1001) 0.484±0.2761) Lamb-H组 7 0.328±0.1091) 0.225±0.0781) 与DSS组比较,1)P<0.01。 表 5 各组小鼠结肠TNF-α、IL-6 mRNA含量的比较
X±S 组别 例数 TNF-α mRNA IL-6 mRNA DSS组 6 1.014±0.183 1.008±0.137 Lamb-L组 6 1.338±0.212 2.043±0.745 Lamb-M组 7 1.963±0.943 2.068±0.463 Lamb-H组 7 2.620±0.6391) 2.608±1.2312) 与DSS组比较,1)P<0.01,2)P<0.05。 表 6 各组小鼠结肠TGF-β、IL-10 mRNA含量的比较
X±S 组别 例数 TGF-β mRNA IL-10 mRNA DSS组 6 1.037±0.301 1.042±0.341 Lamb-L组 6 0.523±0.1721) 0.545±0.0951) Lamb-M组 7 0.600±0.2562) 0.525±0.0411) Lamb-H组 7 0.493±0.1931) 0.460±0.1461) 与DSS组比较,1)P<0.01,2)P<0.05。 -
[1] Eisenstein M. Ulcerative colitis: towards remission[J]. Nature, 2018, 563(7730): S33. doi: 10.1038/d41586-018-07276-2
[2] Michielan A, Dinca R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut[J]. Mediators Inflamm, 2015, 2015: 628157.
[3] 靳丽媛, 司艺玲, 陈光辉. 巨噬细胞表型极化在糖尿病心肌病发生发展和修复过程中的作用[J]. 解放军医学院学报, 2017, 38(1): 68-70, 74.
[4] Levine A, Sigall Boneh R, Wine E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases[J]. Gut, 2018, 67(9): 1726-1738. doi: 10.1136/gutjnl-2017-315866
[5] Sabino J, Torres J. You Are What You Eat, But Can Diet Prevent Inflammatory Bowel Diseases?[J]. Gastroenterology, 2020, 158(8): 2304-2305. doi: 10.1053/j.gastro.2020.04.035
[6] 汪一晗, 邢凤玲, 傅宏阳, 等. 发物对接触性皮炎模型大鼠的影响[J]. 浙江中医药大学学报, 2021, 45(3): 222-228.
[7] Guilleminault L, Williams EJ, Scott HA, et al. Diet and Asthma: Is It Time to Adapt Our Message?[J]. Nutrients, 2017, 9(11): 1227. doi: 10.3390/nu9111227
[8] Zheng HC, Wang YA, Liu ZR, et al. Consumption of Lamb Meat or Basa Fish Shapes the Gut Microbiota and Aggravates Pulmonary Inflammation in Asthmatic Mice[J]. J Asthma Allergy, 2020, 13: 509-520. doi: 10.2147/JAA.S266584
[9] Wu X, Chen H, Gao X, et al. Natural Herbal Remedy Wumei Decoction Ameliorates Intestinal Mucosal Inflammation by Inhibiting Th1/Th17 Cell Differentiation and Maintaining Microbial Homeostasis[J]. Inflamm Bowel Dis, 2022, 28(7): 1061-1071. doi: 10.1093/ibd/izab348
[10] Sun Z, Li J, Dai Y, et al. Indigo Naturalis Alleviates Dextran Sulfate Sodium-Induced Colitis in Rats via Altering Gut Microbiota[J]. Front Microbiol, 2020, 11: 731. doi: 10.3389/fmicb.2020.00731
[11] Sun Z, Li J, Wang W, et al. Qingchang Wenzhong Decoction Accelerates Intestinal Mucosal Healing Through Modulation of Dysregulated Gut Microbiome, Intestinal Barrier and Immune Responses in Mice[J]. Front Pharmacol, 2021, 12: 738152. doi: 10.3389/fphar.2021.738152
[12] 黄美惠, 张志谦, 耿学斯. 溃疡性结肠炎的外科治疗进展[J]. 中国现代普通外科进展, 2020, 23(1): 78-81.
[13] Barmeyer C, Fromm M, Schulzke JD. Active and passive involvement of claudins in the pathophysiology of intestinal inflammatory diseases[J]. Pflugers Arch, 2017, 469(1): 15-26.
[14] Hume DA. The Many Alternative Faces of Macrophage Activation[J]. Front Immunol, 2015, 6: 370.
[15] 方一, 刘倩, 钟捷, 等. 巨噬细胞极化对溃疡性结肠炎病情发展的影响[J]. 诊断学理论与实践, 2015, 14(6): 568-572.
[16] 李跃文, 万强. 绿原酸调控巨噬细胞极化治疗溃疡性结肠炎的机制研究[J]. 浙江中医药大学学报, 2023, 47(9): 969-975, 1001.
[17] 臧超越, 裴明, 李昱芃. 基于"因人制宜"及"发物"理论初探慢性肾脏病"辨体论忌"[J]. 天津中医药, 2022, 39(12): 1564-1567.
[18] 朱飞叶, 谢冠群. 中医饮食禁忌发物探讨[J]. 中华中医药杂志, 2018, 33(7): 3104-3106.
[19] 赵进喜, 贾海忠, 段行武, 等. 发物致病, 多种多样; 趋利避害, 稳定病情[J]. 环球中医药, 2022, 15(1): 42-45.
[20] 崔子烨, 谭妍妍, 丁康. 饮食干预与炎症性肠病相关性的中西医研究进展[J]. 中国中西医结合消化杂志, 2023, 31(2): 151-156. https://zxyxh.whuhzzs.com/article/doi/10.3969/j.issn.1671-038X.2023.02.16
[21] Jowett SL, Seal CJ, Pearce MS, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study[J]. Gut, 2004, 53(10): 1479-1484.
[22] 刘慧敏, 侯慧, 李丹萍. 高摄入红肉通过改变肠道菌群加重小鼠溃疡性结肠炎[J]. 中国微生态学杂志, 2023, 35(4): 396-402.
-




 下载:
下载: