Analysis of influencing factors on the efficacy of tenofovir alafenamide fumarate in the treatment of chronic hepatitis B
-
摘要: 目的 初步探讨在慢性乙型病毒性肝炎(CHB)患者口服富马酸丙酚替诺福韦(TAF)病毒治疗过程中(第24周、48周),影响TAF抗病毒治疗效果的因素。方法 选择2020年4月-2022年1月在门诊或住院的CHB患者为研究对象,回顾性分析其临床资料,根据纳入、排除标准筛选出57例有完整临床资料的CHB患者,分为完全应答组和未完全应答组,运用修正Poissoin回归进行统计分析处理。结果 在抗病毒第24周时,单因素回归中,基线HBV DNA、基线HBsAg、患者之前是否采用过其他抗病毒药物对抗病毒作用差异有统计学意义,多因素回归分析中提示基线HBV DNA差异有统计学意义;在抗病毒治疗第48周时,单因素回归分析提示基线HBV DNA、基线HBsAg差异有统计学意义,多因素回归提示基线HBV DNA差异有统计学意义。结论 CHB患者在口服TAF抗病毒过程中,在第24周、48周时,CHB患者的基线HBV DNA水平可以预测抗病毒的治疗效果。Abstract: Objective To initially investigate the factors influencing the efficacy of tenofovir alafenamide fumarate(TAF) antiviral therapy during the course(24 weeks and 48 weeks) of tenofovir alafenamide fumarate(TAF) viral therapy in patients with chronic viral hepatitis B(CHB patients).Methods Patients with CHB who were outpatients or inpatients in the Hospital between April 2020 and January 2022 were selected as the study subjects, and their clinical data were retrospectively analyzed. According to the inclusion and discharge criteria, a total of 57 CHB patients with complete clinical data were screened into fully responsive and incompletely responsive groups, which were processed for statistical analysis using modified Poissoin regression.Results It shows that there are statistical differences in baseline HBV DNA, baseline HBsAg and whether the patient has used other antiviral drugs before in the univariate regression at the 24th week of antiviral treatment. The multivariate regression analysis shows that there are statistical differences in baseline HBV DNA. At the 48th week of antiviral treatment, the univariate regression analysis shows that there are statistical differences in baseline HBV DNA and baseline HBsAg, Multivariate regression showed that there was a significant difference in baseline HBV DNA.Conclusion It shows that the baseline HBV DNA level of CHB patients can predict the antiviral treatment effect at 24 and 48 weeks.
-
乙型肝炎病毒(HBV)已经感染了全球近2.5亿人,而不同的地区发病率有所不同,我国是慢性乙型病毒性肝炎(CHB)流行的中高风险地区,表面抗原阳性率约为6.0%,统计仍有2000万~3000万CHB患者[1-2]。研究显示,若CHB患者不能进行有效的抗病毒治疗,若干年后就会出现肝硬化、肝癌等情况。研究显示我国约有50%的肝癌和HBV直接有关[3]。所以有效的抗病毒治疗对于我国CHB患者的生命健康至关重要。
目前对于CHB患者,推荐使用富马酸丙酚替诺福韦(TAF)、富马酸替诺福韦二吡呋酯(TDF)、恩替卡韦(ETV)作为一线口服药物[4]。研究显示这些药物对HBV复制有良好的抑制作用,具有改善肝脏组织学的作用,可降低肝癌的发生率[5]。其中TAF是替诺福韦(TFV)的前药,可减轻血浆暴露风险,降低对肾功能的影响[6],TAF比TDF具有更强的血浆稳定性、安全性。
既往研究同时发现HBV DNA水平、基线ALT水平、HBeAg状态(阴性/阳性)、基线HBsAg水平等可能都会影响TDF、ETV的治疗效果[7-8]。但目前国内对于影响TAF抗病毒治疗效果因素的文章较少,所以本文初步探讨在CHB患者口服TAF抗病毒治疗过程中,影响TAF抗病毒治疗效果的因素,为临床医师提供更适合的治疗方案。
1. 资料与方法
1.1 临床资料
选择2020年4月—2022年1月在天津中医药大学第一附属医院门诊或住院的CHB患者为研究对象,回顾性分析其临床资料,纳入的368例CHB患者曾在我院口服TAF抗病毒治疗,根据纳入、排除标准,共有57例CHB患者具有完整的临床资料,其中男46例,女11例,年龄34~51岁。
1.2 研究方法
通过医院电子病例系统回顾收集口服TAF抗病毒治疗的CHB患者,初步探讨在CHB患者口服TAF抗病毒治疗过程中(0~24周、0~48周)影响TAF抗病毒治疗效果的因素,即影响达到完全病毒学应答的因素(HBV DNA < 20 IU/mL)。
将57例患者分为两组:完全应答组和未完全应答组,通过构建多因素回归的方法,探讨CHB患者口服TAF在治疗到第24周和第48周时可能影响TAF抗病毒治疗效果的因素。
1.3 纳入及排除标准
纳入标准:①符合《慢性乙型肝炎防治指南(2019年版)》CHB诊断标准并具有抗病毒治疗指征;②在随访期间,患者口服TAF单药治疗;③临床资料相对完整,疗程至少48周。
排除标准:①失代偿期肝硬化患者;②原发及继发的肝癌患者;③合并酒精性肝病、药物性肝病、其他病毒性感染等其他原因引起的肝病;④患有肾脏、心血管疾病等其他系统疾病。
1.4 检测方法
HBV DNA检测采用雅培试剂,现将检测结果统一定义,当HBV DNA < 20 IU/mL或检测不到时,即抗病毒治疗后获得了完全病毒学应答,同时将HBV DNA的检测结果 < 20 IU/mL或检测不到时的DNA结果统一记录为10 IU/mL。HBsAg的正常范围为0~0.05 IU/mL,HBeAg正常范围为0~1.0 S/CO;肝脏生物化学指标的检测,其中ALT的正常值上限为55 U/L。
1.5 统计学方法
使用SPSS 21.0版本软件对收集数据进行统计分析。正态分布的计量资料以X±S表示,非正态分布的计量资料以M(P25,P75)表示,计数资料以例数和%表示,以P < 0.05为差异有统计学意义。将基线HBV DNA、HBsAg的数据进行以10为底的对数转换,同时运用修正Poisson回归探讨可能影响达到完全病毒学应答的因素,运用GraphPad Prism 9.0版本对统计分析结果进行绘图。
2. 结果
2.1 一般情况
共有57例患者纳入研究,HBeAg阴性患者有23例,HBeAg阳性患者有34例。在第24周时共有26例CHB患者完全病毒学应答,完全病毒学应答率为45.6%,第48周时共有31例CHB患者实现完全病毒学应答,完全病毒学应答率为54.4%。
2.2 第24周完全病毒学应答统计分析结果
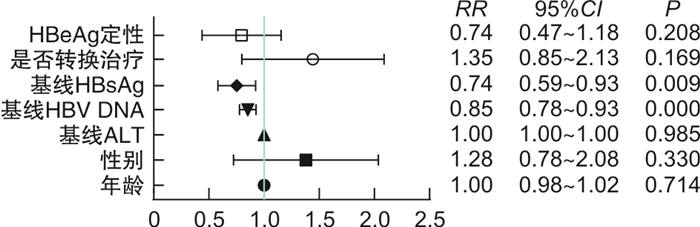
在CHB患者口服TAF抗病毒治疗到第24周时,通过单因素回归分析,基线HBV DNA滴度对是否能达到完全病毒学应答差异有统计学意义(RR=0.76,95%CI 0.68~0.86,P < 0.001),CHB患者在使用TAF之前是否使用过其他抗病毒药物对第24周是否完全病毒学应答差异有统计学意义(RR=2.02,95%CI 2.14~3.57,P=0.016)。基线HBsAg水平对是否能达到完全病毒学应答差异有统计学意义(RR=0.73,95%CI 0.57~0.94,P=0.013),而CHB患者年龄、性别、基线ALT水平、HBeAg定性情况(阴性/阳性)对治疗到第24周时是否能达到完全病毒学应答,差异无统计学意义(图 1)。
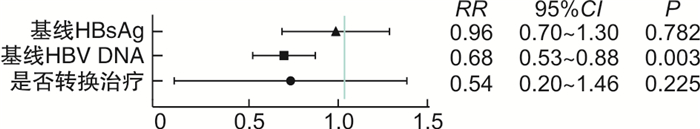
在CHB患者口服TAF抗病毒治疗到第24周时,通过构建多因素回归分析,基线HBV DNA滴度对第24周是否达到完全病毒学应答差异有统计学意义(RR=0.68,95%CI 0.53~0.88,P=0.003),而CHB患者年龄、性别、基线HBsAg水平、基线ALT水平、患者HBeAg定性情况、CHB患者使用TAF之前是否使用过其他抗病毒药物的情况对第24周是否能达到完全病毒学应答,差异无统计学意义(图 2)。
2.3 第48周完全病毒学应答统计分析结果
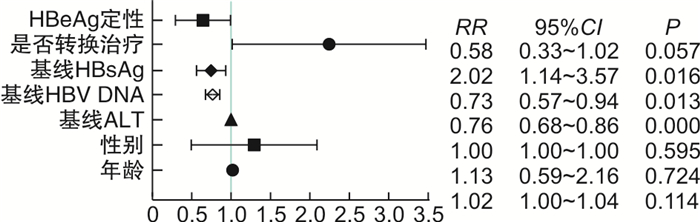
在CHB患者口服TAF抗病毒治疗到第48周时,通过构建单因素回归分析,基线HBV DNA滴度对48周是否能达到完全病毒学应答差异有统计学意义(RR=0.85,95%CI 0.78~0.93,P < 0.001),基线HBsAg水平对第48周是否完全病毒学应答差异有统计学意义(RR=0.74,95%CI 0.59~0.93,P=0.009)。而患者年龄、性别、基线ALT水平、患者HBeAg定性情况、患者使用TAF之前是否使用过其他抗病毒药物的情况对第48周是否达到完全病毒学应答,差异无统计学意义。见图 3。
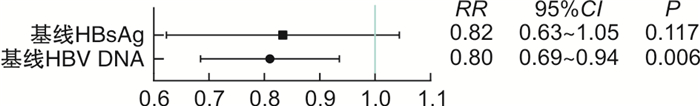
在CHB患者口服TAF抗病毒治疗到第48周时,通过构建多因素回归分析,基线HBV DNA滴度对第48周是否能达到完全病毒学应答差异有统计学意义(RR=0.80,95%CI 0.69~0.94,P=0.006),而CHB患者的年龄、性别、基线HBsAg水平、基线ALT水平、患者HBeAg定性情况、CHB患者使用TAF之前是否使用过其他抗病毒药物的情况对第48周是否能到达完全病毒学应答差异无统计学意义。见图 4。
3. 讨论
TAF在2018年被正式引入我国,相对于ETV、TDF等其他抗病毒药物,TAF在两项Ⅲ期试验中显示了较好的抗病毒治疗效果、更好的肾脏、骨密度安全性[9-10],但国内对于影响TAF抗病毒疗效因素的研究较少[11],本文初步探究可能影响病毒学应答的因素。
既往研究提示CHB患者基线HBeAg定性情况、基线HBsAg水平、基线ALT水平、HBV DNA水平等可以作为抗病毒药物治疗效果的预测因素[12-15]。研究发现虽然HBe Ag可能是预测因素之一,并不参与HBV病毒复制过程,但可影响机体特异性免疫应答的发挥,因此HBeAg可能是影响抗病毒作用的原因之一[16-18]。在干扰素治疗CHB患者时,有学者发现HBsAg水平的下降趋势可以预测聚乙二醇化干扰素(Peg IFN-α)治疗CHB患者的病毒学应答的情况[19],同时其他研究发现对于接受NAs治疗的患者,血清HBsAg水平也可独立预测口服NAs进行治疗的CHB患者的病毒学应答情况[20]。部分研究提示,治疗前ALT水平可能也影响抗病毒治疗效果,考虑治疗前ALT更高的患者可能自身的免疫清除力更强,在抗病毒治疗后更容易达到完全病毒学抑制[11, 21]。基线HBV DNA水平是预测抗病毒治疗效果的因素,治疗前HBV水平更低的患者更能获得病毒学应答[8, 22]。
本研究在多因素回归中发现CHB患者治疗前的HBV DNA水平越高,口服TAF抗病毒的治疗效果相对更差,考虑与机体对病毒清除力较低有关[23-24]。在本研究中并未发现HBeAg定性、患者之前是否采用过其他抗病毒药物、基线ALT水平、基线HBsAg水平等影响TAF抗病毒治疗效果。考虑本组研究入组例数较少,随访时间只到达了48周,目前CHB患者需要长期口服抗病毒药物,从而实现长期完全病毒学应答甚至临床治愈的情况,这需要纳入更多的CHB患者、随访更长的时间来观察是否有其他的影响病毒学应答的因素。
综上所述,TAF具有较好的抗病毒治疗作用及较高的病毒学应答率,相对于TDF有更好的肾脏、骨密度安全性[25-26],但由于无法清除肝细胞体内的cccDNA环,仍需长期口服[27-28]。本研究显示CHB患者治疗前HBV DNA水平可能是预测治疗第24周、48周完全病毒学应答的独立预测因子。
利益冲突 所有作者均声明不存在利益冲突
-
[1] Razavi-Sheare D, Gamkrelidze I, Nguyen MH, et. al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study[J]. Lancet Gastroenterol Hepatol, 2018, 3(6): 383-403.
[2] Liu J, Liang W, Jing W, et. al. Countdown to 2030: eliminating hepatitis B disease, China[J]. Bull World Health Organ, 2019, 97(3): 230-238.
[3] Rongshou Z, Chunfeng Q, Siwei Z, et. al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030[J]. Chin J Cancer Res, 2018, 30(6): 571-579.
[4] 王贵强, 王福生, 庄辉, 等. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35(12): 2648-2669.
[5] 丁惠国, 屠红, 曲春枫, 等. 原发性肝癌的分层筛查与监测指南(2020版)[J]. 临床肝胆病杂志, 2021, 37(2): 286-295. doi: 10.3969/j.issn.1001-5256.2021.02.009
[6] 鲁俊锋, 侯维, 马丽娜, 等. 富马酸丙酚替诺福韦初始治疗慢性乙型肝炎患者的早期疗效[J]. 中国肝脏病杂志(OL), 2020, 12(3): 29-33.
[7] 林畅琪. 慢性乙型肝炎患者发生病毒学突破和病毒学应答的影响因素分析[D]. 广州: 广东药科大学, 2019.
[8] 周珲堃, 江建宁, 苏明华, 等. 恩替卡韦与替诺福韦酯治疗高病毒载量慢性乙型肝炎患者的效果分析[J]. 临床肝胆病杂志, 2022, 38(3): 532-536. doi: 10.3969/j.issn.1001-5256.2022.03.008
[9] Chan HL, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg positive chronic hepatitis B virus infection: a randomised, doubleblind, phase 3, non-inferiority trial[J]. Lancet Gastroenterol Hepatol, 2016, 1(3): 185-195.
[10] Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial[J]. Lancet Gastroenterol Hepatol, 2016, 1(3): 196-206.
[11] 任天棋, 杨倩倩, 马鹤铭, 等. TAF治疗慢性乙型肝炎的研究进展[J]. 肝脏, 2019, 24(11): 1319-1322. doi: 10.3969/j.issn.1008-1704.2019.11.037
[12] 肖芷琪, 周福元, 周彬, 等. 慢性乙型肝炎患者的基线丙氨酸氨基转移酶水平与抗病毒疗效的关系[J]. 南方医科大学学报, 2019, 39(2): 150-155.
[13] 周珲堃, 江建宁, 苏明华, 等. 恩替卡韦与替诺福韦酯治疗高病毒载量慢性乙型肝炎患者的效果分析[J]. 临床肝胆病杂志, 2022, 38(3): 532-536. doi: 10.3969/j.issn.1001-5256.2022.03.008
[14] Hu P, Shang J, Zhang W, et al. HBsAg loss with peg-interferon alfa-2a in hepatitis B patients with partial response to nucleos (t) ide analog: new switch study[J]. J Clin Transl Hepatol, 2018, 6(1): 25-34.
[15] 傅维佳, 李成忠. 核苷(酸)类药物长期治疗HBeAg阳性慢性乙型肝炎患者血清HBsAg、HBeAg和HBV DNA动态变化及应答相关性[J]. 肝脏, 2018, 23(2): 114-117.
[16] 王培培. 慢性乙型肝炎患者恩替卡韦治疗后病毒学应答的影响因素分析[J]. 中国实用医刊, 2021, 48(13): 60-64.
[17] 李明慧, 胡蕾苹, 张璐, 等. HBEAG阳性慢性乙型肝炎用核苷(酸)类似物治疗部分病毒学应答患者转换聚乙二醇干扰素α-2A治疗的研究[J]中华肝脏病杂志, 2015, 23(11): 826-831. doi: 10.3760/cma.j.issn.1007-3418.2015.11.006
[18] 阎文昭, 张岩, 刘英辉, 等. 血清HBsAg和HBeAg定量检测对HBeAg阳性慢性乙型肝炎患者病毒学应答的预测价值[J]. 国际流行病学传染病学杂志, 2016, 43(3): 177-181.
[19] 白一春, 邓霁红, 梅小平. 影响聚乙二醇干扰素α-2B治疗慢性乙型肝炎患者疗效的多因素分析[J]. 实用肝脏病杂志, 2019, 22(4): 490-493. doi: 10.3969/j.issn.1672-5069.2019.04.010
[20] 吕月, 安子英, 李艳伟. 等. HBSAG/抗-HBS双阳性慢性乙型肝炎患者的临床和病毒学特点[J]. 中国病毒病杂志, 2017, 7(1): 23-26.
[21] Wang Q, Hu L, Ding D, et al. Upgrade Combination Response Is Limited by Prolonged Nucelos(t)ide Analogue Therapy in HBeAg-positive Chronic Hepatitis B: A Real-life Study[J]. J Clin Transl Hepatol, 2018, 6(1): 11-17.
[22] 陈琦琪, 程澄, 王京京, 等. 恩替卡韦应答不佳的HBeAg阳性慢性乙型肝炎患者的基线特征研究[J]. 中华实验和临床病毒学杂志, 2019(4): 415-418.
[23] Terrault NA, Lok AF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67(4): 1560-1599.
[24] 周子凡. HBeAg阳性慢性乙型肝炎患者恩替卡韦治疗48周病毒学不完全应答早期预测因素研究[D]. 沈阳: 中国医科大学, 2021.
[25] 王素娜, 连建奇, 贾战生, 等. 富马酸丙酚替诺福韦治疗慢性乙型肝炎的研究现状[J]. 临床肝胆病杂志, 2019, 35(8): 1828-1833.
[26] 胡璇, 李彤, 王玉珊, 等. 丙酚替诺福韦治疗慢性乙型肝炎的临床研究进展[J]. 胃肠病学和肝病学杂志, 2021, 30(8): 950-954.
[27] 朱云竹. 富马酸丙酚替诺福韦对慢性乙型肝炎初治及经治患者的疗效及安全性分析[D]. 合肥: 安徽医科大学, 2022.
[28] European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67(2): 370-398.
期刊类型引用(1)
1. 王秀英,刘亚利,李晓洁,王少珊,林莉,杨珍珍,吴库生. 富马酸丙酚替诺福韦片的健康人体生物等效性研究. 汕头大学医学院学报. 2023(04): 205-210 .  百度学术
百度学术
其他类型引用(3)
-




 下载:
下载: