To explore the mechanism of metabolic instability in the pathogenesis of ulcerative colitis based on the Zhuo Du theory
-
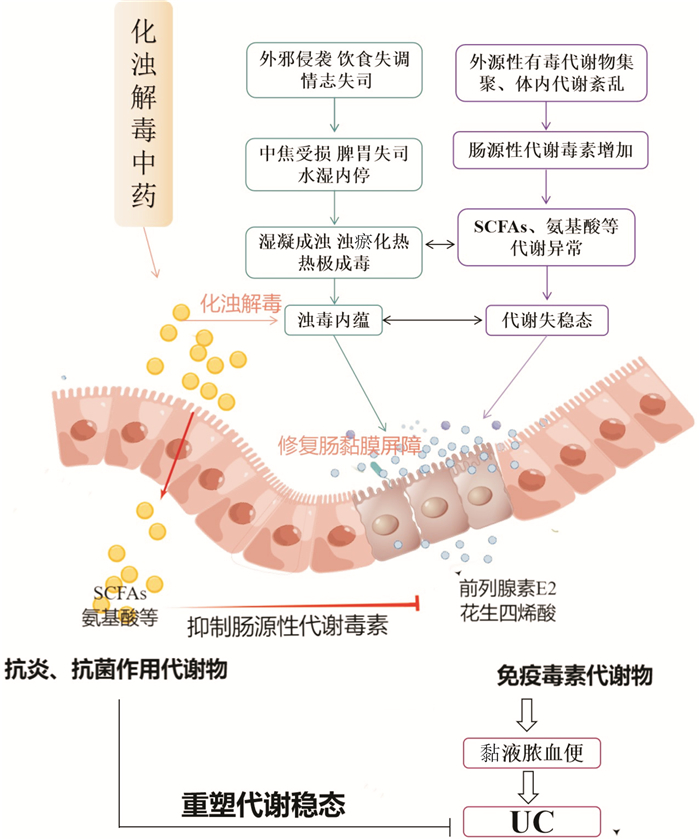
摘要: 溃疡性结肠炎患者普遍存在代谢失稳态。代谢失稳态不仅是驱动溃疡性结肠炎发生的原动力,同时也是溃疡性结肠炎致病的必然结果,故改善代谢失稳态具有重要意义。浊毒内蕴是溃疡性结肠炎发病的关键病机,贯穿溃疡性结肠炎发病的整个过程。代谢失稳态所导致的毒性代谢物释放、肠黏膜失完整性、炎症浸润及免疫失衡状态与浊毒内蕴病机表征相契合。化浊解毒法是重塑溃疡性结肠炎代谢稳态的重要治则,通过借助化浊解毒药物清除肠道污秽,重塑肠道代谢环境。本文以代谢失稳态为切入点,探讨溃疡性结肠炎浊毒内蕴的实质,为化浊解毒法治疗溃疡性结肠炎的相关研究提供思路。Abstract: Metabolic instability is common in patients with ulcerative colitis. Metabolic instability is not only the driving force of ulcerative colitis, but also the inevitable result of ulcerative colitis. Therefore, it is important to improve metabolic instability. Zhuo Du internalization is the key pathogenesis of ulcerative colitis, which runs through the whole process of ulcerative colitis. The release of toxic metabolites, intestinal mucosal integrity, inflammatory infiltration and immune imbalance caused by metabolic instability are consistent with the pathogenesis of Zhuo Du internalization. Turbidity and detoxification method is an important treatment for remolding the metabolic homeostasis of ulcerative colitis, which can remove intestinal filth with the help of turbidity and detoxification drugs, and then, the intestinal metabolic environment can be remade. Based on the metabolic instability, this paper discusses the essence of turbid ulcerative colitis toxin, and provides some ideas for the research on the treatment of ulcerative colitis with turbidity and detoxification method.
-

-
[1] Lv Q, Xing Y, Liu J, et al. Lonicerin targets EZH2 to alleviate ulcerative colitis by autophagy-mediated NLRP3 inflammasome inactivation[J]. Acta Pharm Sin B, 2021, 11(9): 2880-2899. doi: 10.1016/j.apsb.2021.03.011
[2] Retnakumar SV, Muller S. Pharmacological Autophagy Regulators as Therapeutic Agents for Inflammatory Bowel Diseases[J]. Trends Mol Med, 2019, 25(6): 516-537. doi: 10.1016/j.molmed.2019.03.002
[3] Du L, Ha C. Epidemiology and Pathogenesis of Ulcerative Colitis[J]. Gastroenterol Clin North Am, 2020, 49(4): 643-654. doi: 10.1016/j.gtc.2020.07.005
[4] Crotty B. Ulcerative colitis and xenobiotic metabolism[J]. Lancet, 1994, 343(8888): 35-38. doi: 10.1016/S0140-6736(94)90882-6
[5] Kreuter R, Wankell M, Ahlenstiel G, et al. The role of obesity in inflammatory bowel disease[J]. Biochim Biophys Acta Mol Basis Dis, 2019, 1865(1): 63-72. doi: 10.1016/j.bbadis.2018.10.020
[6] Zhang Y, Feng D, Zeng Y, et al. Xuedan Sustained Release Pellets Ameliorate Dextran Sulfate Sodium-Induced Ulcerative Colitis in Rats by Targeting Gut Microbiota and MAPK Signaling Pathways[J]. Front Pharmacol, 2022, 13: 833972. doi: 10.3389/fphar.2022.833972
[7] 李佃贵, 杨倩, 才艳茹, 等. 李佃贵教授中西医结合治疗溃疡性结肠炎经验[J]. 中国中西医结合消化杂志, 2019, 27(4): 244-246. https://zxyxh.whuhzzs.com/article/doi/10.3969/j.issn.1671-038X.2019.04.01
[8] Schilling CH, Letscher D, Palsson BO. Theory for the systemic definition of metabolic pathways and their use in interpreting metabolic function from a pathway-oriented perspective[J]. J Theor Biol, 2000, 203(3): 229-248. doi: 10.1006/jtbi.2000.1073
[9] Deberardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us?[J]. Cell, 2012, 148(6): 1132-1144. doi: 10.1016/j.cell.2012.02.032
[10] Jones JG. Hepatic glucose and lipid metabolism[J]. Diabetologia, 2016, 59(6): 1098-1103. doi: 10.1007/s00125-016-3940-5
[11] Org E, Blum Y, Kasela S, et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort[J]. Genome Biol, 2017, 18(1): 70. doi: 10.1186/s13059-017-1194-2
[12] 江彬, 赵文涛, 欧阳聪, 等. 细胞代谢调控网络[J]. 厦门大学学报(自然科学版), 2022, 61(3): 346-364.
[13] 王彬, 周欢, 章清华, 等. 基于肠道微生态探讨代谢综合征之"浊毒"病机[J]. 北京中医药大学学报, 2019, 42(5): 374-377.
[14] Hyun CK. Molecular and Pathophysiological Links between Metabolic Disorders and Inflammatory Bowel Diseases[J]. Int J Mol Sci, 2021, 22(17): 9139. doi: 10.3390/ijms22179139
[15] Harper JW, Zisman TL. Interaction of obesity and inflammatory bowel disease[J]. World J Gastroenterol, 2016, 22(35): 7868-7881. doi: 10.3748/wjg.v22.i35.7868
[16] Michalak A, Mosinska P, Fichna J. Common links between metabolic syndrome and inflammatory bowel disease: Current overview and future perspectives[J]. Pharmacol Rep, 2016, 68(4): 837-846. doi: 10.1016/j.pharep.2016.04.016
[17] Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment[J]. Science, 2013, 342(6161): 967-970. doi: 10.1126/science.1240527
[18] Zheng X, Zhao A, Xie G, et al. Melamine-induced renal toxicity is mediated by the gut microbiota[J]. Sci Transl Med, 2013, 5(172): 122r-172r.
[19] Khan I, Ullah N, Zha L, et al. Alteration of Gut Microbiota in Inflammatory Bowel Disease(IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome[J]. Pathogens, 2019, 8(3): 126-152. doi: 10.3390/pathogens8030126
[20] Federici M. Gut microbiome and microbial metabolites: a new system affecting metabolic disorders[J]. J Endocrinol Invest, 2019, 42(9): 1011-1018. doi: 10.1007/s40618-019-01022-9
[21] Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease[J]. Nat Rev Gastroenterol Hepatol, 2020, 17(4): 223-237. doi: 10.1038/s41575-019-0258-z
[22] 伍晓涵, 刘占举. 溃疡性结肠炎最新发病机制的认识[J]. 中国中西医结合消化杂志, 2023, 31(4): 237-242. https://zxyxh.whuhzzs.com/article/doi/10.3969/j.issn.1671-038X.2023.04.01
[23] 张娇娇, 张帆, 余星星, 等. 溃疡性结肠炎发病机制及中西医治疗研究进展[J]. 辽宁中医药大学学报, 2021, 23(1): 70-74.
[24] 杨杰, 张丽曼, 王丽丽, 等. 清肠化瘀方治疗溃疡性结肠炎疗效及对IDO、Treg/Th17平衡的影响[J]. 广州中医药大学学报, 2023, 40(7): 1642-1648.
[25] 王杰, 隗鑫, 陈威, 等. 代谢组学技术在中药复方配伍规律研究中的应用[J]. 中草药, 2022, 53(5): 1528-1539.
[26] Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice[J]. Diabetes, 2008, 57(6): 1470-1481.
[27] 刘德华, 孙宝林. 胃肠道微生物种群与人类消化系统疾病相关性研究进展[J]. 生物学杂志, 2019, 36(4): 1-6.
[28] Bjerrum JT, Wang Y, Hao F, et al. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn's disease and healthy individuals[J]. Metabolomics, 2015, 11: 122-133.
[29] Yu M, Wang Q, Ma Y, et al. Aryl Hydrocarbon Receptor Activation Modulates Intestinal Epithelial Barrier Function by Maintaining Tight Junction Integrity[J]. Int J Biol Sci, 2018, 14(1): 69-77.
[30] Furumatsu K, Nishiumi S, Kawano Y, et al. A role of the aryl hydrocarbon receptor in attenuation of colitis[J]. Dig Dis Sci, 2011, 56(9): 2532-2544.
[31] Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease[J]. Nat Commun, 2018, 9(1): 3294.
[32] Kim CH. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids[J]. Cell Mol Immunol, 2021, 18(5): 1161-1171.
[33] Hu J, Lin S, Zheng B, et al. Short-chain fatty acids in control of energy metabolism[J]. Crit Rev Food Sci Nutr, 2018, 58(8): 1243-1249.
[34] Tedelind S, Westberg F, Kjerrulf M, et al. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease[J]. World J Gastroenterol, 2007, 13(20): 2826-2832.
[35] Shen S, Prame KK, Wen SW, et al. Deficiency of Dietary Fiber Modulates Gut Microbiota Composition, Neutrophil Recruitment and Worsens Experimental Colitis[J]. Front Immunol, 2021, 12: 619366.
[36] Huang X, Oshima T, Tomita T, et al. Butyrate Alleviates Cytokine-Induced Barrier Dysfunction by Modifying Claudin-2 Levels[J]. Biology(Basel), 2021, 10(3): 205.
[37] 张纨, 孙建慧, 李娅, 等. 国医大师李佃贵治疗溃疡性结肠炎经验[J]. 中华中医药杂志, 2019, 34(4): 1504-1506.
[38] 娄莹莹, 李佃贵, 霍永利, 等. 溃疡性结肠炎特色病机"浊毒损膜伤络"及其意义[J]. 中国中西医结合杂志, 2022, 42(6): 749-753.
[39] 吴开春, 梁洁, 冉志华, 等. 炎症性肠病诊断与治疗的共识意见(2018年·北京)[J]. 中国实用内科杂志, 2018, 38(9): 796-813.
[40] Sivaprakasam S, Bhutia YD, Yang S, et al. Short-Chain Fatty Acid Transporters: Role in Colonic Homeostasis[J]. Compr Physiol, 2017, 8(1): 299-314.
[41] Russo E, Giudici F, Fiorindi C, et al. Immunomodulating Activity and Therapeutic Effects of Short Chain Fatty Acids and Tryptophan Post-biotics in Inflammatory Bowel Disease[J]. Front Immunol, 2019, 10: 2754.
[42] 于媛媛, 祝溢阳, 张世诚, 等. 植物多糖对胃肠道疾病的治疗作用研究进展[J]. 中华中医药学刊, 2024, 42(4): 194-199.
[43] 王正品, 李佃贵, 杜艳茹, 等. 浊毒致病论与现代中医病因学[J]. 中医杂志, 2010, 51(1): 11-13.
[44] 段雨婷. 基于肠道菌群代谢物SCFAs-GPR41/43-MAPK通路探究茯苓多糖对肠黏膜损伤的保护作用[D]. 合肥: 安徽中医药大学, 2023.
[45] Qu C, Yuan ZW, Yu XT, et al. Patchouli alcohol ameliorates dextran sodium sulfate-induced experimental colitis and suppresses tryptophan catabolism[J]. Pharmacol Res, 2017, 121: 70-82. http://www.xueshufan.com/publication/2609027890
[46] Hu J, Huang H, Che Y, et al. Qingchang Huashi Formula attenuates DSS-induced colitis in mice by restoring gut microbiota-metabolism homeostasis and goblet cell function[J]. J Ethnopharmacol, 2021, 266: 113394.
[47] Yuan Z, Yang L, Zhang X, et al. Mechanism of Huang-lian-Jie-du decoction and its effective fraction in all eviating acute ulcerative colitis in mice: Regulating arachidonic acid metabolism and glycerophospholipid metabolism[J]. J Ethnopharmacol, 2020, 259: 112872.
-

计量
- 文章访问数: 77
- 施引文献: 0



 下载:
下载: