Cordycepin alleviates necrotic apoptosis of colonic tissue in ulcerative colitis rats by inhibiting RIP1/RIP3/MLKL signaling pathway
-
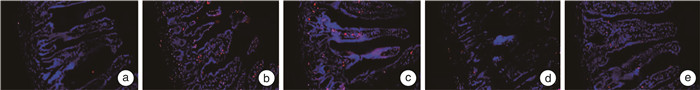
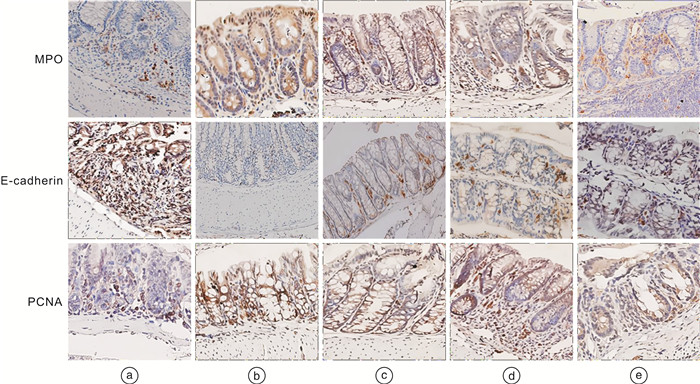
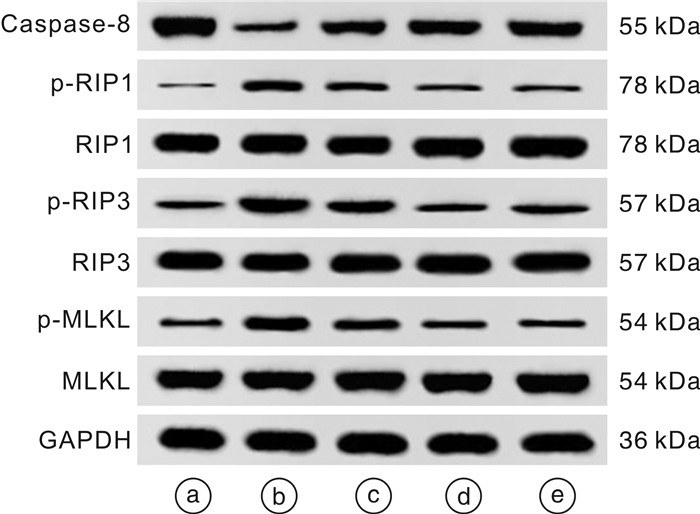
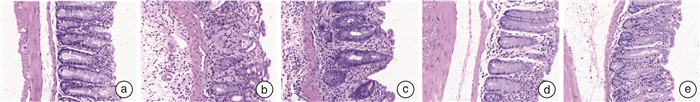
摘要: 目的 探讨虫草素通过抑制受体相互作用蛋白(receptor interacting protein,RIP)1/RIP3/混合谱系激酶结构域样蛋白(mixed lineage kinase domain like protein,MLKL)信号通路减轻溃疡性结肠炎(ulcerative colitis,UC)大鼠结肠组织坏死性凋亡的作用机制。方法 采用三硝基苯磺酸(TNBS)和乙醇联合灌肠法制造UC模型大鼠,成功造模后将56只大鼠随机分为模型组、虫草素低剂量组(40 mg/kg)、虫草素高剂量组(80 mg/kg)、Nec-1组(RIP1抑制剂,0.6 mg/kg),每组14只;另取14只大鼠作为对照组。大鼠给予相应药物干预,1次/d,连续2周。给药结束24 h后,记录大鼠一般行为状态并进行疾病活动指数(disease activity index,DAI)评分;苏木精-伊红染色观察大鼠结肠组织病理学变化;ELISA法检测大鼠血清IL-1β、IL-6、肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)水平;TUNEL检测大鼠结肠组织细胞凋亡率;免疫组化法检测大鼠结肠组织髓过氧化物酶(myeloperoxidase,MPO)、E-钙黏蛋白(epithelial cadherin,E-cadherin)、增殖细胞核抗原(proliferating cell nuclear antigen,PCNA)阳性表达;蛋白印迹法检测大鼠结肠组织半胱氨酸蛋白酶-8(Caspase-8)、RIP1、磷酸化RIP1(p-RIP1)、RIP3、磷酸化RIP3(p-RIP3)、MLKL、磷酸化MLKL(p-MLKL)蛋白的表达。结果 对照组大鼠大便正常,结肠黏膜结构完整,未见溃疡和糜烂,固有层腺体排列紧密,层次清晰,上皮细胞排列规则未见坏死变性;与对照组比较,模型组大鼠体重下降,出现稀便、血便,结肠黏膜结构破坏明显,固有层腺体排列紊乱,间隙变宽,上皮细胞明显减少,炎性细胞浸润明显,DAI评分、血清IL-1β、IL-6、TNF-α、结肠组织细胞凋亡率、MPO、PCNA阳性表达、p-RIP1/RIP1、p-RIP3/RIP3、p-MLKL/MLKL蛋白比值均显著升高(均P < 0.05);结肠组织E-cadherin阳性表达、Caspase-8蛋白表达均显著降低(均P < 0.05)。与模型组比较,虫草素低、高剂量组大鼠结肠组织病理损伤程度依次减轻,DAI评分、血清IL-1β、IL-6、TNF-α、结肠组织细胞凋亡率、MPO、PCNA阳性表达、p-RIP1/RIP1、p-RIP3/RIP3、p-MLKL/MLKL蛋白比值依次降低(P < 0.05);结肠组织E-cadherin阳性表达、Caspase-8蛋白表达依次升高(P < 0.05)。Nec-1组与虫草素高剂量组大鼠结肠组织病理学变化程度及各项指标比较均差异无统计学意义(P>0.05)。结论 虫草素能够通过抑制RIP1/RIP3/MLKL信号通路减轻UC大鼠结肠炎症,减少结肠组织细胞坏死性凋亡。
-
关键词:
- 虫草素 /
- 受体相互作用蛋白 /
- 混合谱系激酶结构域样蛋白 /
- 溃疡性结肠炎 /
- 凋亡
Abstract: Objective To investigate the mechanism of cordycepin alleviating necrotic apoptosis of colonic tissue in ulcerative colitis(UC) rats by inhibiting receptor interacting protein(RIP)1/RIP3/mixed lineage kinase domain like protein(MLKL) signaling pathway.Methods Using trinitrobenzene sulfonic acid(TNBS) and ethanol as a combined enema method to create UC model rats, 56 successfully modeled rats were randomly divided into model group, cordycepin low(40 mg/kg), high(80 mg/kg) dose groups, and Nec-1(RIP1 inhibitor, 0.6 mg/kg) group, with 14 rats in each group; another 14 rats were taken as control group; rats in each group were given corresponding drug intervention, once a day, for two consecutive weeks. After 24 hours of administration, the general behavioral status of the rats was recorded and the disease activity index(DAI) score was calculated; HE staining was used to observe the histopathological changes of the colon of rats; detection of serum interleukin(IL)-1β, IL-6, tumor necrosis factor(TNF)-α levels in rats by ELISA; TUNEL was used to detect the apoptosis rate of colon tissue in rats; immunohistochemical method was used to detect the positive expression of myeloperoxidase(MPO), epithelial cadherin(E-cadherin), and proliferating cell nuclear antigen(PCNA) in the colon tissue of rats; the expression of cysteine proteinase-8(Caspase-8), RIP1, phosphorylated RIP1(p-RIP1), RIP3, phosphorylated RIP3(p-RIP3), MLKL, and phosphorylated MLKL(p-MLKL) proteins in rat colon tissue were detected by Western blotting.Results In the control group, the rats had normal bowel movements, complete colonic mucosa structure, no ulcers and erosion were observed, lamina propria glands were closely arranged with clear layers, and epithelial cells were arranged regularly without necrosis and degeneration; compared with control group, the body mass of rats in the model group was decreased, there were loose stools and bloody stools, the structure of colon mucosa was damaged obviously, the arrangement of glands in lamina propria was disordered, the gap was widened, epithelial cells were significantly reduced, and inflammatory cell infiltration was obvious, DAI score, serum IL-1β, IL-6, TNF-α, apoptosis rate, positive expression of MPO and PCNA, p-RIP1/RIP1, p-RIP3/RIP3, p-MLKL/MLKL protein ratios in colon tissue were significantly increased(P < 0.05), E-cadherin positive expression and Caspase-8 protein expression in colon tissue were significantly decreased(P < 0.05); compared with model group, the pathological damage degree of colonic tissue of rats in cordycepin low and high dose groups decreased successively, DAI score, serum IL-1β, IL-6, TNF-α, apoptosis rate, positive expression of MPO and PCNA, p-RIP1/RIP1, p-RIP3/RIP3, p-MLKL/MLKL protein ratios in colon tissue were decreased in turn(P < 0.05), E-cadherin positive expression and Caspase-8 protein expression in colon tissue were increased in turn(P < 0.05); there were no significant differences in the degree of colonic histopathological changes and indexes between the Nec-1 group and the cordycepin high dose group(P>0.05).Conclusion Cordycepin can reduce the inflammation of rats with UC by inhibiting RIP1/RIP3/MLKL signaling pathway and reduce the necrotizing apoptosis of colonic histiocytes. -

-
表 1 各组大鼠血清IL-1β、IL-6、TNF-α水平的比较
pg/mL,X ± S 组别 例数 IL-1β IL-6 TNF-α 对照组 14 24.35±4.03 98.41±15.02 84.34±12.15 模型组 14 64.01±8.141) 192.25±27.821) 175.21±25.021) 虫草素低剂量组 14 53.07±7.542) 154.21±18.012) 148.45±21.412) 虫草素高剂量组 14 39.21±6.322)3) 115.47±16.412)3) 112.35±18.012)3) Nec-1组 14 38.35±6.452)3) 118.74±17.462)3) 108.45±16.412)3) 与对照组比较,1)P < 0.05;与模型组比较,2)P < 0.05;与虫草素低剂量组比较,3)P < 0.05。 表 2 各组大鼠结肠组织MPO、E-cadherin、PCNA阳性表达的比较
%,X ± S 组别 例数 MPO E-cadherin PCNA 对照组 14 8.51±2.10 82.94±9.35 10.04±2.12 模型组 14 74.58±15.591) 2.51±0.401) 77.55±15.551) 虫草素低剂量组 14 51.49±12.882) 18.94±2.052) 65.27±11.882) 虫草素高剂量组 14 28.27±7.942)3) 45.55±5.362)3) 51.58±10.572)3) Nec-1组 14 26.96±8.292)3) 47.48±5.402)3) 50.16±10.222)3) 与对照组比较,1)P < 0.05;与模型组比较,2)P < 0.05;与虫草素低剂量组比较,3)P < 0.05。 表 3 各组大鼠结肠组织Caspase-8、p-RIP1/RIP1、p-RIP3/RIP3、p-MLKL/MLKL表达的比较
X ± S 组别 例数 Caspase-8/(GAPDH) p-RIP1/RIP1 p-RIP3/RIP3 p-MLKL/MLKL 对照组 14 0.96±0.12 0.16±0.03 0.29±0.05 0.22±0.04 模型组 14 0.41±0.051) 0.53±0.081) 0.84±0.141) 0.61±0.121) 虫草素低剂量组 14 0.64±0.072) 0.41±0.102) 0.62±0.112) 0.44±0.082) 虫草素高剂量组 14 0.82±0.082)3) 0.25±0.052)3) 0.37±0.092)3) 0.32±0.062)3) Nec-1组 14 0.85±0.092)3) 0.24±0.052)3) 0.39±0.092)3) 0.29±0.052)3) 与对照组比较,1)P < 0.05;与模型组比较,2)P < 0.05;与虫草素低剂量组比较,3)P < 0.05。 -
[1] El-Zimaity H, Shaffer SR, Riddell RH, et al. Beyond Neutrophils for Predicting Relapse and Remission in Ulcerative Colitis[J]. J Crohns Colitis, 2023, 17(5): 767-776. doi: 10.1093/ecco-jcc/jjac178
[2] Wang Z, Yang L, Sun S. Effect of Intestinal Microbiota Transplantation on Intestinal Flora and Inflammatory Factor Levels in Patients with Ulcerative Colitis[J]. Infect Drug Resist, 2023, 16(1): 1183-1191.
[3] Yan X, Yu X, Jiang C, et al. Tonifying-Qi-and-Detoxification Decoction attenuated injuries of colon and lung tissues in ulcerative colitis rat model via regulating NF-κB and p38MAPK pathway[J]. Ann Transl Med, 2022, 10(8): 455-467. doi: 10.21037/atm-22-892
[4] Asgharzadeh F, Yaghoubi A, Nazari SE, et al. The beneficial effect of combination therapy with sulfasalazine and valsartan in the treatment of ulcerative colitis[J]. Excli J, 2021, 20(1): 236-247.
[5] Ino S, Yano T, Kuno A, et al. Nuclear translocation of MLKL enhances necroptosis by a RIP1/RIP3-independent mechanism in H9c2 cardiomyoblasts[J]. J Pharmacol Sci, 2023, 151(2): 134-143. doi: 10.1016/j.jphs.2022.12.009
[6] Zhu J, Xin M, Xu C, et al. Ligand-based substituent-anchoring design of selective receptor-interacting protein kinase 1 necroptosis inhibitors for ulcerative colitis therapy[J]. Acta Pharm Sin B, 2021, 11(10): 3193-3205. doi: 10.1016/j.apsb.2021.05.017
[7] Lin QS, Chen P, Wang WX, et al. RIP1/RIP3/MLKL mediates dopaminergic neuron necroptosis in a mouse model of Parkinson disease[J]. Lab Invest, 2020, 100(3): 503-511. doi: 10.1038/s41374-019-0319-5
[8] Guo Y, Wu X, Wu Q, et al. Dihydrotanshinone, a natural product, ameliorates DSS-induced experimental ulcerative colitis in mice[J]. Toxicol Appl Pharmacol, 2018, 344(1): 35-45.
[9] Lan T, Yu Y, Zhang J, et al. Cordycepin Ameliorates Nonalcoholic Steatohepatitis by Activation of the AMP-Activated Protein Kinase Signaling Pathway[J]. Hepatology, 2021, 74(2): 686-703. doi: 10.1002/hep.31749
[10] Wei P, Wang K, Luo C, et al. Cordycepin confers long-term neuroprotection via inhibiting neutrophil infiltration and neuroinflammation after traumatic brain injury[J]. J Neuroinflammation, 2021, 18(1): 137-148. doi: 10.1186/s12974-021-02188-x
[11] Kopalli SR, Cha KM, Cho JY, et al. Cordycepin from Medicinal Fungi Cordyceps militaris Mitigates Inflammaging-Associated Testicular Damage via Regulating NF-κB/MAPKs Signaling in Naturally Aged Rats[J]. Mycobiology, 2022, 50(1): 89-98.
[12] 王钰嘉, 于千惠, 卢雨微, 等. 穴位埋线联合艾灸对溃疡性结肠炎大鼠结肠组织IL-6/JAK/STAT3信号通路的影响[J]. 针刺研究, 2022, 47(6): 525-530. https://www.cnki.com.cn/Article/CJFDTOTAL-XCYJ202206009.htm
[13] 鲍楠, 吴冰, 张蓬杰, 等. 虫草素对慢性肾脏病大鼠血管内皮损伤的保护作用及其机制[J]. 现代生物医学进展, 2022, 22(5): 818-821, 856. https://www.cnki.com.cn/Article/CJFDTOTAL-SWCX202205004.htm
[14] 王楠楠, 梁映霞, 张蕊, 等. 细胞程序性坏死抑制剂Nec-1可减轻神经病理性痛大鼠的痛觉过敏[J]. 临床麻醉学杂志, 2019, 35(7): 697-700. https://www.cnki.com.cn/Article/CJFDTOTAL-LCMZ201907022.htm
[15] 罗敏, 杜英杰, 姜燕诗, 等. 基于内质网应激PERK信号通路探讨芍药汤对UC大鼠的作用机制[J]. 湖南中医药大学学报, 2021, 41(11): 1663-1668. https://www.cnki.com.cn/Article/CJFDTOTAL-HNZX202111004.htm
[16] 陈双兰, 刘青松, 张怡, 等. 基于细胞信号通路探讨中药复方治疗溃疡性结肠炎的研究进展[J]. 中华中医药学刊, 2023, 41(1): 107-111. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYHS202301025.htm
[17] Min Y, Ding Y, Huang Q, et al. Cordycepin inhibited the retinoblastoma cell proliferation, migration, and invasion as well as lung metastasis via modulating c-Myc/cyclin D1 pathway[J]. Chem Biol Drug Des, 2023, 101(3): 605-613.
[18] Ku CW, Ho TJ, Huang CY, et al. Cordycepin Attenuates Palmitic Acid-Induced Inflammation and Apoptosis of Vascular Endothelial Cells through Mediating PI3K/Akt/eNOS Signaling Pathway[J]. Am J Chin Med, 2021, 49(7): 1703-1722.
[19] Li C, Xu Y, Gao T, et al. Ruxolitinib Alleviates Inflammation, Apoptosis, and Intestinal Barrier Leakage in Ulcerative Colitis via STAT3[J]. Inflamm Bowel Dis, 2023, 29(8): 1191-1201.
[20] Feng Z, Zhou P, Wu X, et al. Hydroxysafflor yellow A protects against ulcerative colitis via suppressing TLR4/NF-κB signaling pathway[J]. Chem Biol Drug Des, 2022, 99(6): 897-907.
[21] Alattar A, Alshaman R, Al-Gayyar MMH. Therapeutic effects of sulforaphane in ulcerative colitis: effect on antioxidant activity, mitochondrial biogenesis and DNA polymerization[J]. Redox Rep, 2022, 27(1): 128-138.
[22] Vazquez-Carretero MD, García-Miranda P, Balda MS, et al. Proper E-cadherin membrane location in colon requires Dab2 and it modifies by inflammation and cancer[J]. J Cell Physiol, 2021, 236(2): 1083-1093.
[23] Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death[J]. Cell Mol Immunol, 2021, 18(5): 1106-1121.
[24] Zhou H, Zhou M, Hu Y, et al. TNF-α Triggers RIP1/FADD/Caspase-8-Mediated Apoptosis of Astrocytes and RIP3/MLKL-Mediated Necroptosis of Neurons Induced by Angiostrongylus cantonensis Infection[J]. Cell Mol Neurobiol, 2022, 42(6): 1841-1857.
[25] Marino-Merlo F, Klett A, Papaianni E, et al. Caspase-8 is required for HSV-1-induced apoptosis and promotes effective viral particle release via autophagy inhibition[J]. Cell Death Differ, 2023, 30(4): 885-896.
-

计量
- 文章访问数: 235
- 施引文献: 0



 下载:
下载: